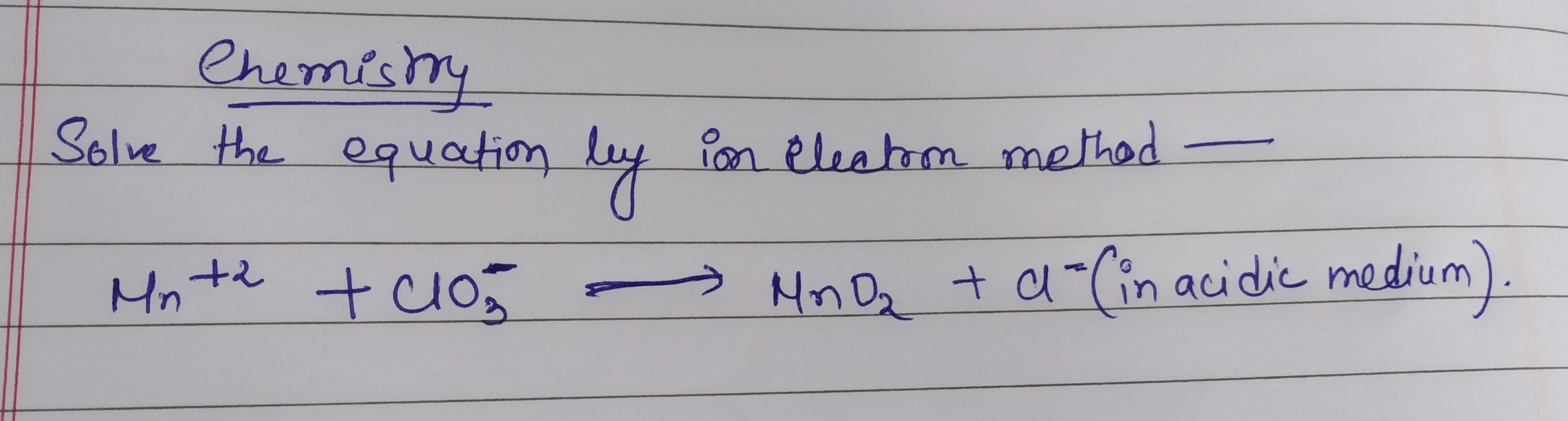
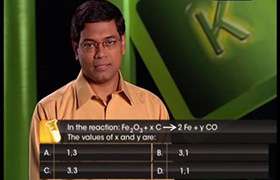CBSE Class 11-science Answered
1. Find out the element which undergoes a change in oxidation number:
The oxidation number of Mg increases from 0 to +2 in Mg metal to Mg(NO3)2 and N decrease from +5 in HNO3 to +1 in N2O
2. Find out the total increase/decrease in oxidation number :
Total Increase in Mg is 2 and decrease in N is 4 but there are two N atoms in N2O and one in HNO3. Hence, the decrease in Oxidation number of N is 2 X 4 =8
3. Balance increase/decrease in Oxidation number:
Since the total increase is 2 and decrease is 8.thereore, multiply Mg on LHS by 4 and combine step 2 and 3
Hence equation will be written as :
4 Mg(aq) +2 HNO3(aq) → Mg(NO3)2 (aq) + N2O (g)+ H2O
4. Balance all atoms other than O and H:
To balance Mg on either side, multiply (MgNO3) by 4 we have,
4 Mg +2HNO3 → 4 Mg (NO3)2+N2O+H2O
Now there are 10 N atoms on RHS and 2 on LHS, therefore to balance N atoms change the coefficients of HNO3 from 2 to 10 on LHS and we have,
4 Mg(NO3)2+10HNO3 → 4 Mg (NO3)2+N2O+H2O
5. Balance O and H :
There are 30 O atoms on LHS but only 26 atoms on RHS, therefore, to balance O atoms, change the coefficients of H2O from 1 to 5. We have,
4 Mg(NO3)2+2HNO3 → 4 Mg (NO3)2+N2O+5H2O
Here H atoms get balanced automatically.