CBSE Class 10 Answered
- Change of colour
Certain chemical reactions are characterised by a change in colour of the reactants.
Examples:
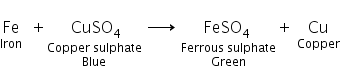
i. When few pieces of iron are dropped into a blue-coloured copper sulphate solution, the blue colour of the solution fades and turns light green.
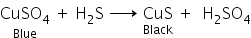
ii. When blue-coloured copper sulphate reacts with hydrogen sulphide gas, black-coloured copper sulphide is formed.
- Formation of precipitates
Certain chemical reactions are characterised by the formation of insoluble solid substances called precipitates.
Examples:
i. When a solution of silver nitrate is added to a solution of sodium chloride, a white insoluble substance (precipitate) of silver chloride is formed.
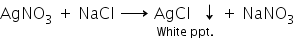
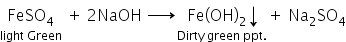
ii. When ferrous sulphate solution is added to sodium hydroxide, a dirty green precipitate of ferrous hydroxide is formed.
iii. Lead nitrate decomposes to give yellow lead monoxide, brown nitrogen dioxide and colourless oxygen.











