CBSE Class 10 Answered
Maam
1. Can you give me the details of all oxides of iron - its colour,formation,chemical equation,etc?
2. Can there be two or more products in a combination reaction? Give examples.
Asked by sreejuu | 18 May, 2019, 12:52: PM
1.
Oxides of IRON:
Fe(II) oxides
1) FeO
2) FeO2
Fe(III) oxides:
Fe2O3
Preparation:
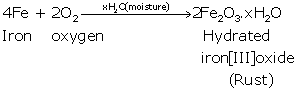
Colour ranges from reddish to brown.
2. Yes, there is only one product is formed at the end of combination reactions.
Combination Reaction :
A chemical reaction in which two or more substances combine to form a single product is called a combination reaction or synthesis.
When iron and sulphur are heated together, they combine to form a single product, iron sulphide.
2Fe(s) + S(s) → FeS (s)
Iron Sulphur Iron sulphide
Answered by Ramandeep | 18 May, 2019, 01:40: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by 09.10bjanvhijadhav | 02 Mar, 2024, 08:22: AM
CBSE 10 - Chemistry
Asked by prassanna.j | 01 Mar, 2024, 11:59: AM
CBSE 10 - Chemistry
Asked by susrisangita792 | 07 Jun, 2023, 11:54: AM
CBSE 10 - Chemistry
Asked by nehashekh291 | 02 Jan, 2023, 07:26: PM
CBSE 10 - Chemistry
Asked by satulurivictor | 17 Dec, 2022, 08:51: PM
CBSE 10 - Chemistry
Asked by Shadm4242 | 20 Aug, 2022, 12:35: PM
CBSE 10 - Chemistry
Asked by shyjukadakkavur | 12 Jun, 2022, 08:38: PM
CBSE 10 - Chemistry
Asked by pachchigarkeyur | 08 Mar, 2022, 12:05: PM
CBSE 10 - Chemistry
Asked by krrishsahu371 | 01 Dec, 2021, 09:22: AM
CBSE 10 - Chemistry
Asked by alintareji | 22 Aug, 2021, 08:11: PM










