CBSE Class 11-science Answered
Kolbe's Electrolytic method..
Asked by sc.sallan | 26 Nov, 2009, 08:05: PM
Free radical mechanism has been proposed for Kolbe’s reaction. For example by, Kolbe’s electrolytic method, electrolysis of aqueous solution of potassium acetate gives ethane. At anode acetate ion forms acetate free radical. This free radical undergoes decarboxylation to form methyl free radical. This methyl free radical then dimerises to form ethane. Methane cannot be prepared by this method because the alkane formed in this reaction, is formed by combination of two alkyl free radicals. Therefore it would contain at least two carbon atoms. Thus alkane with even number of carbon atoms would only be formed.
.jpg)
Answered by | 27 Nov, 2009, 04:20: PM
Concept Videos
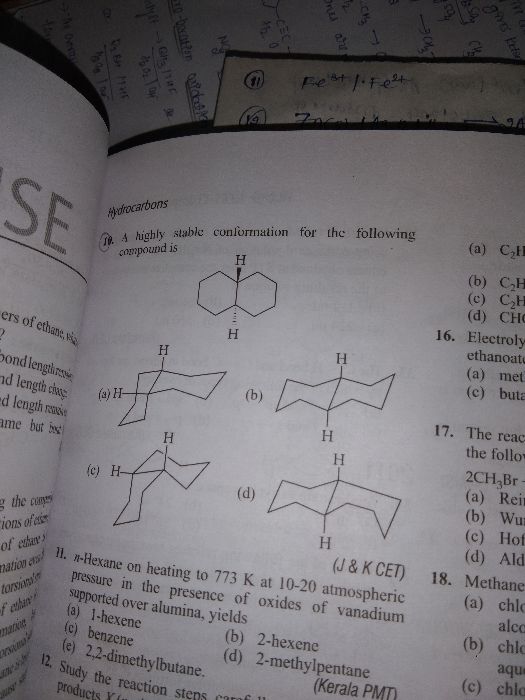
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM