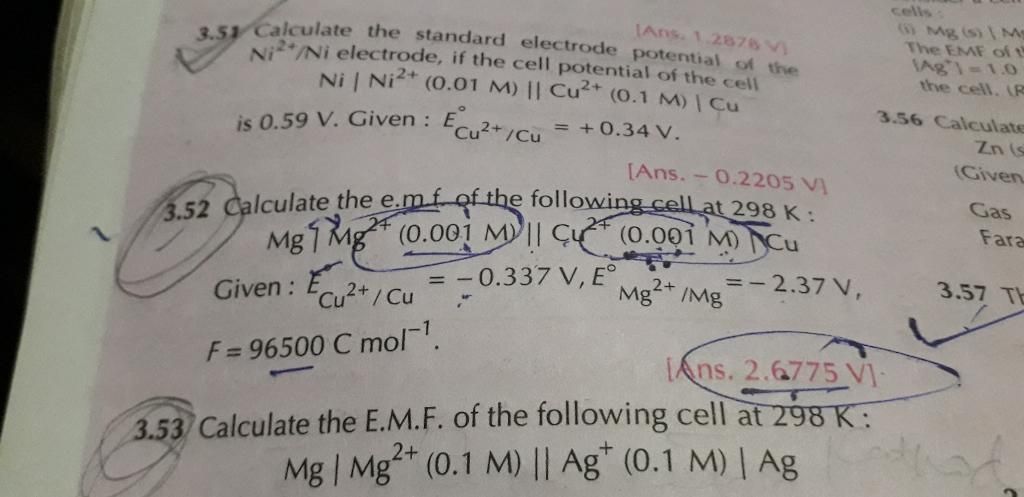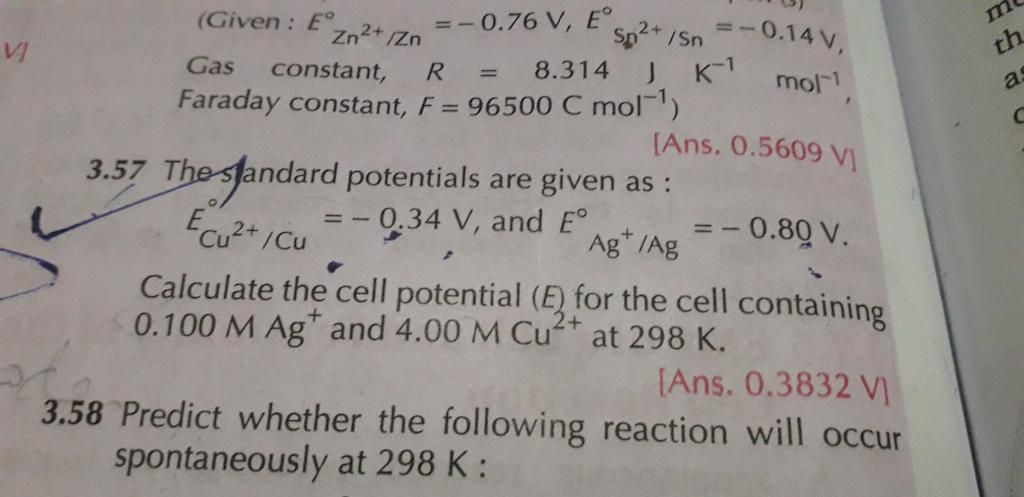CBSE Class 12-science Answered
Kindly give detailed answers to the following questions.
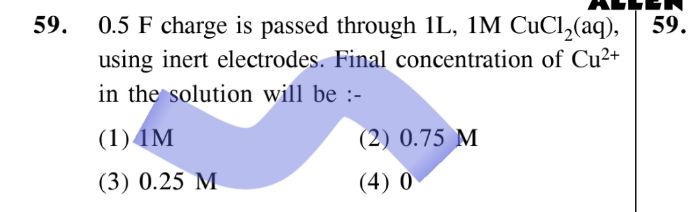
24 all parts

Asked by Varsneya Srinivas | 24 Dec, 2017, 09:58: AM
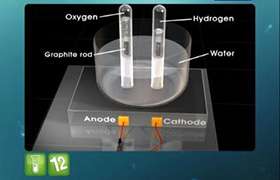
a) As silver is deposited on the electrode from AgNO3 solution,
Ag+ + e- → Ag(s) ................................reduction
Atomic mass of Ag = 107.8u
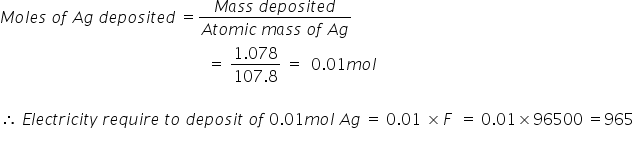
b) 2 H2O → O2 + 4H+ 4e-
4 moles of electrons are required to deposit 1 mol O2
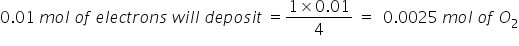
Therefore, mols of O2 librated = moles χ molar mass = 0.0025 Χ 32 = 0.08 gm
Answered by Ramandeep | 25 Dec, 2017, 01:53: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 05:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 04:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 08:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 09:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:11: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Jul, 2019, 09:49: PM