CBSE Class 8 Answered
karthik took three test tubes 'x','y','z'with zinc sulphate solution in all the test tubes .hedropped a piece of copper ,aluminium,iron in each tube .in which testtube does the reaction takes place.why?
write balanced equation for it?
Asked by arajeevshashank | 11 Aug, 2018, 03:30: PM
When a piece of copper is dipped in the solution of ZnSO4 there will be no reaction because according to the reactivity series the copper is below the zinc hence it is less reactive to displace zinc from zinc sulphate.
When a piece of aluminium is dipped in the solution of ZnSO4, according to the reactivity series aluminium is more reactive than zinc hence it displaces zinc from its salt solution. the reaction takes place as
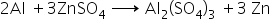
When a piece of iron is dipped in the solution of ZnSO4, no reaction takes place because according to reactivity series iron is less reactive than zinc.
Answered by Ramandeep | 11 Aug, 2018, 07:29: PM
Application Videos
Concept Videos
CBSE 8 - Science
Asked by ayanking905 | 17 Feb, 2023, 11:49: AM
CBSE 8 - Science
Asked by jashanpreetsingh9567 | 29 May, 2021, 07:11: AM
CBSE 8 - Science
Asked by atharva.lad24 | 08 Nov, 2019, 10:48: PM
CBSE 8 - Science
Asked by rajubarman | 10 Jul, 2019, 09:24: AM
CBSE 8 - Science
Asked by arajeevshashank | 11 Aug, 2018, 03:30: PM
CBSE 8 - Science
Asked by rijukurakar | 04 Jul, 2018, 10:18: PM
CBSE 8 - Science
Asked by Topperlearning User | 30 Apr, 2014, 03:18: PM
CBSE 8 - Science
Asked by Topperlearning User | 30 Apr, 2014, 03:17: PM
CBSE 8 - Science
Asked by Topperlearning User | 30 Apr, 2014, 03:13: PM
CBSE 8 - Science
Asked by Topperlearning User | 30 Apr, 2014, 03:16: PM




