CBSE Class 11-science Answered
jkk

Asked by saswati509 | 03 May, 2020, 10:10: AM
Given:
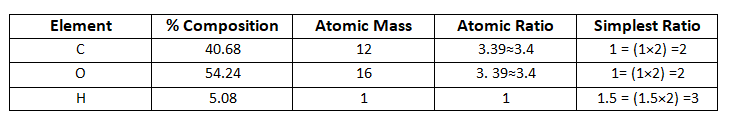
Pecentage composition:
C= 40.68%
H= 5.08%
O = 100 - 45.76
O = 54.24%
Empirical formula is C2H3O2
Empirical formula mass : 24+ 3+ 32 = 59
Molecular mass = 2× Vapour density
= 2 ×59 = 118
We konw,
n = 

= 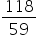
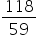
n = 2
Molecular formula = n × Empirical formula
= 2(C2H3O2)
Molecular formula = C4H6O4
Answered by Varsha | 04 May, 2020, 07:05: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM