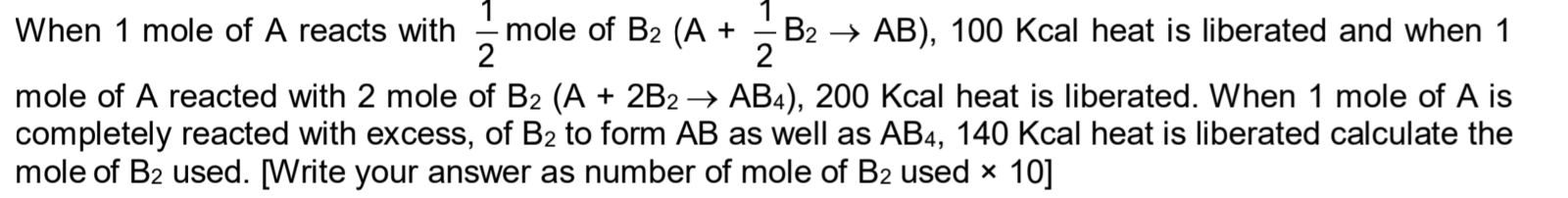CBSE Class 11-science Answered
is their any limitation of significant figures if not why
Asked by pannalal.malakar | 22 Jul, 2014, 04:20: PM
- Significant figure does not convey the magnitude of the reading uncertainty for any specific scale.
- Significant figure neglect the operator error, systematic error which occurs during the real experiments so there will be more uncertainty than what the significant figure of the measurements would suggest.
Answered by Arvind Diwale | 23 Jul, 2014, 01:01: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 10:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 03:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 10:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 03:58: PM
CBSE 11-science - Chemistry
Asked by pb_ckt | 19 May, 2019, 11:56: PM