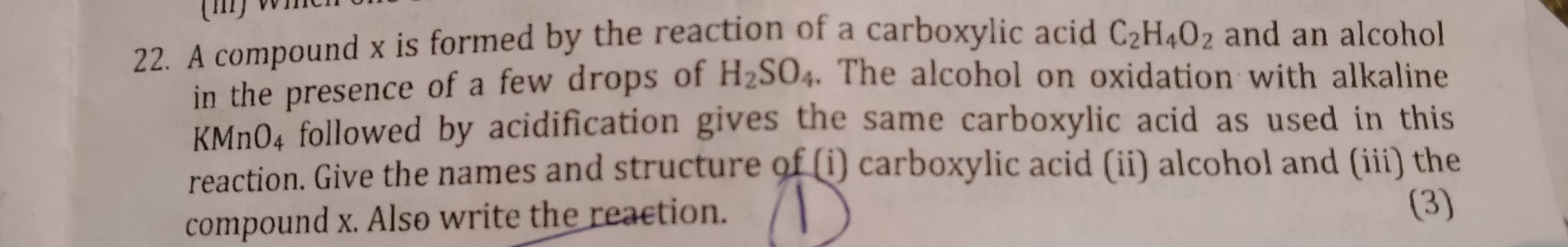CBSE Class 10 Answered
Is saponification exothermic or endothermic?
Asked by B P | 18 Mar, 2017, 08:51: AM
- During the saponification process, as the lye (a strongly alkaline solution, especially of potassium hydroxide, used for washing or cleansing) reacts with the various soap making ingredients, soap (and glycerin) is produced.
- The saponification process itself is an endothermic reaction, meaning that it absorbs heat from the surroundings.
- This “heat stage” of soap making is commonly called as gel phase.
Answered by Prachi Sawant | 20 Mar, 2017, 10:21: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 07:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 03:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 11:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 08:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 05:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 10:07: PM