CBSE Class 12-science Answered
is only free radical chlorination and bromination possible that is isnt free radical iodisation possible and viable??
Asked by Viswanath | 15 Apr, 2015, 08:10: PM
The activation energy is even lower in the chain initiation of alkane iodination.
The complete chain propagation and the first reaction is very endothermic.
So the radical iodination of alkanes does not take place.
Answered by Vaibhav Chavan | 16 Apr, 2015, 11:17: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 01:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 05:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 04:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 10:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 01:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 02:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 08:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:34: PM




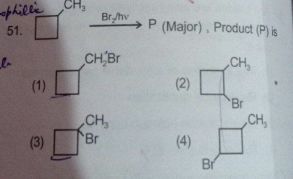
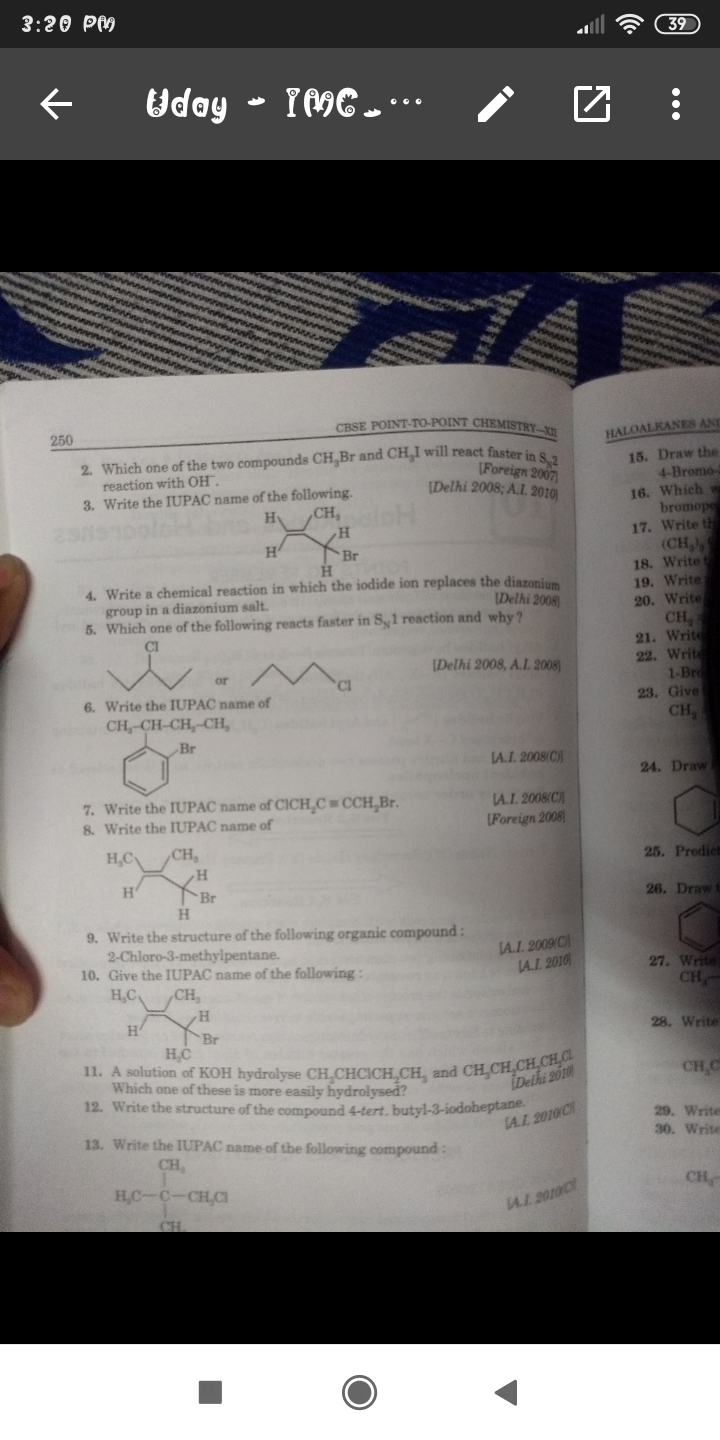
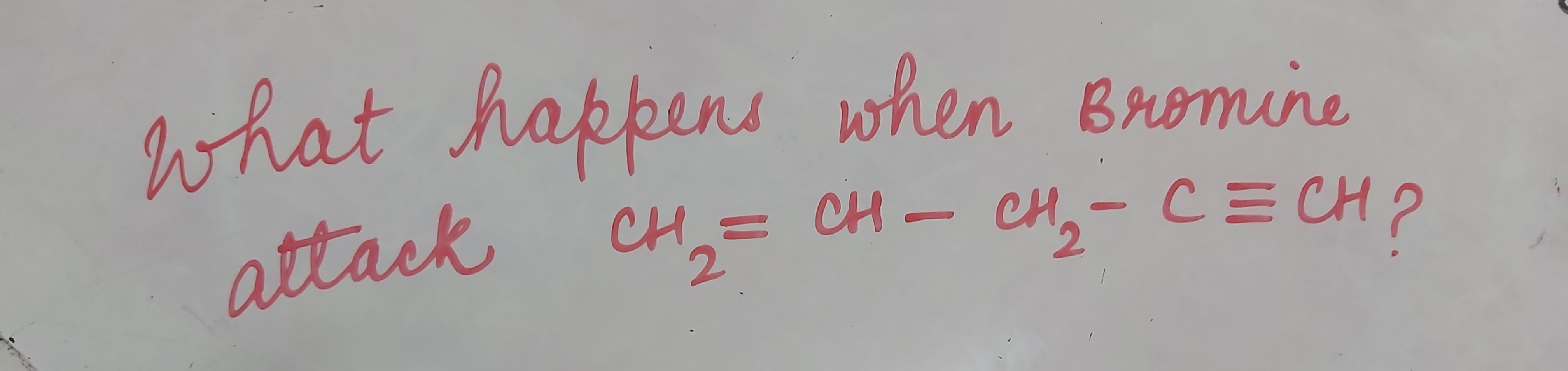
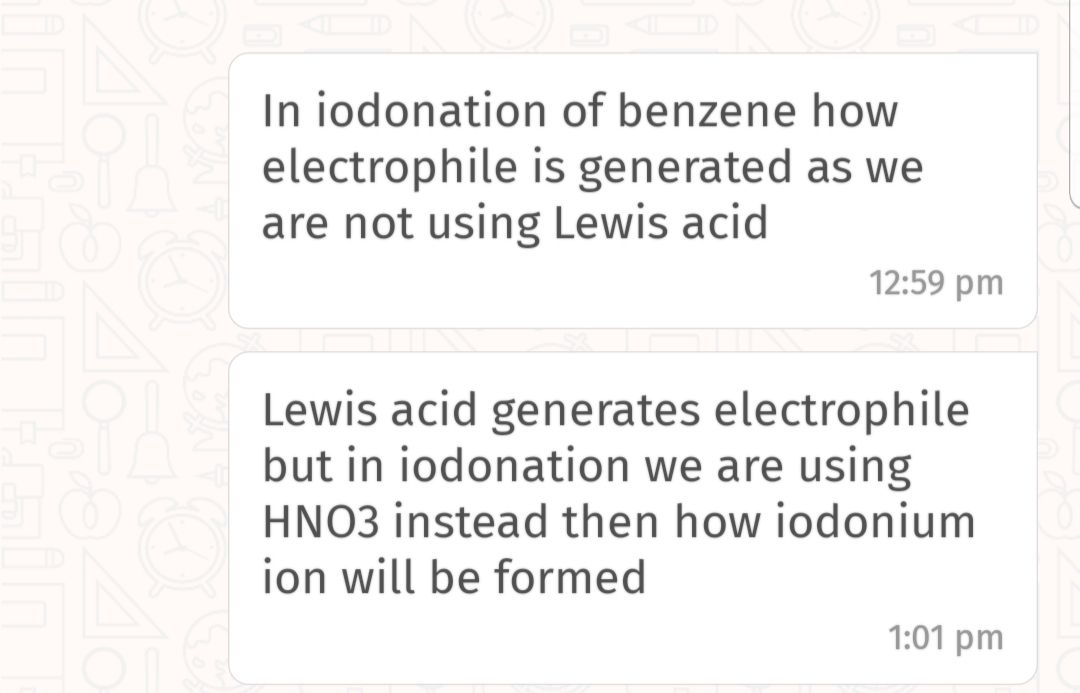
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-