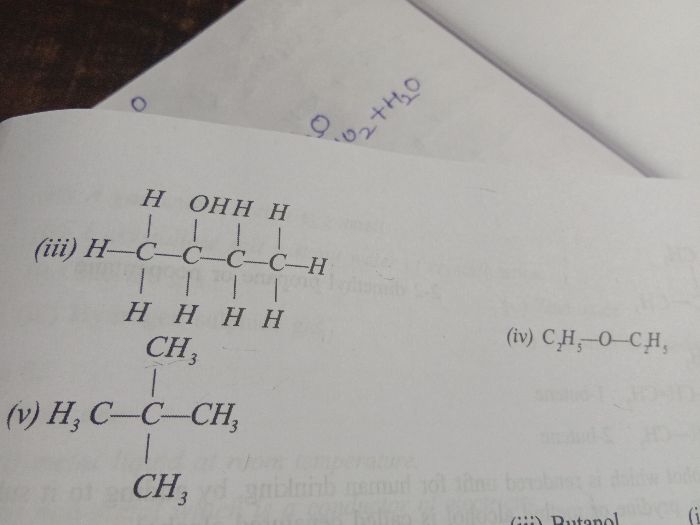ICSE Class 10 Answered
Is carbon a single element to showunique nature like tetravalency ,self linking(catenation) and multiple bonds
Asked by lovemaan5500 | 29 Oct, 2017, 06:07: AM
The carbon has unique property to form bonds with other carbon atoms to give large molecules. The compounds formed may have long chains of carbon, carbon atoms bonded in a ring or branched chains of carbon.
This property of catenation is shown by other elements also but carbon exhibit these propeties to maximum extent.
Due to its smaller size, electronic configuration and maximum bond energy that is greater strength of  bonds, carbon has tendency to form multiple bonds.
bonds, carbon has tendency to form multiple bonds.
 bonds, carbon has tendency to form multiple bonds.
bonds, carbon has tendency to form multiple bonds.Due to small size, carbon has strong tendency to form multiple bond with other carbon atoms,oxygen atoms or nitrogen atoms.
Answered by Varsha | 30 Oct, 2017, 11:50: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by anshpatel6307 | 18 Mar, 2022, 09:32: PM
ICSE 10 - Chemistry
Asked by pritijpjain | 13 Feb, 2021, 07:44: AM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 09:23: AM
ICSE 10 - Chemistry
Asked by arpitt682 | 01 Oct, 2019, 03:44: PM
ICSE 10 - Chemistry
Asked by vijay.prag | 14 Jul, 2019, 09:35: AM
ICSE 10 - Chemistry
Asked by jaiswalsindhuli717 | 16 Dec, 2018, 05:20: PM
ICSE 10 - Chemistry
Asked by hy666333 | 12 Dec, 2018, 08:04: PM
ICSE 10 - Chemistry
Asked by jaiswalsindhuli717 | 12 Nov, 2018, 08:29: PM
ICSE 10 - Chemistry
Asked by jaiswalsindhuli717 | 12 Nov, 2018, 08:18: PM
ICSE 10 - Chemistry
Asked by dr_pradip27121972 | 03 Jun, 2018, 12:55: PM