CBSE Class 10 Answered
Is 1-methyl propane a structural isomer of C4H10(butane)?
 CH2-CH2-CH3
CH2-CH2-CH3
CH3
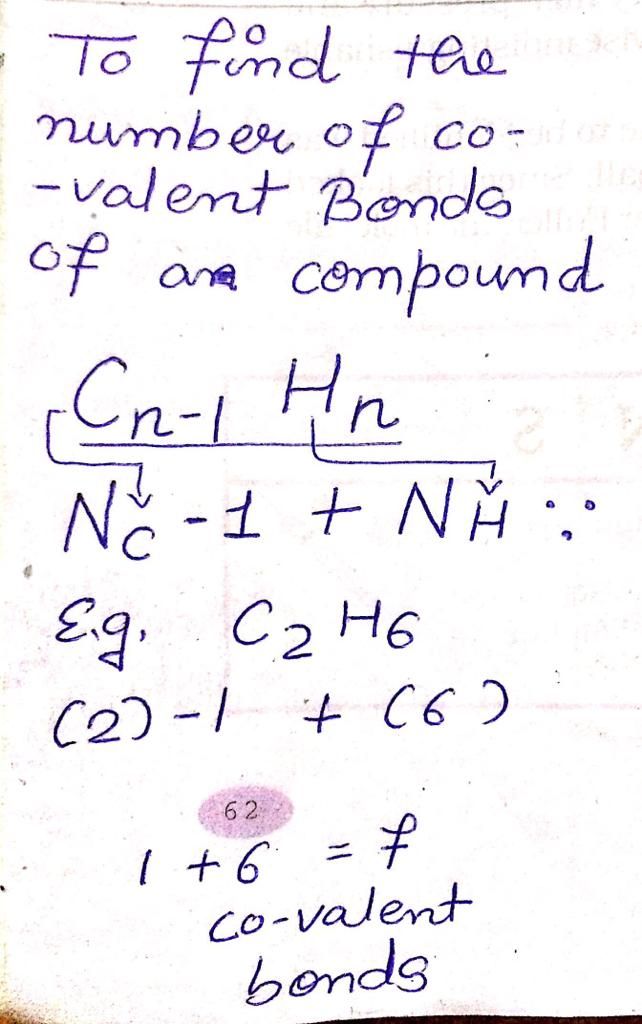
Where do we follow the alphabetical system of nomenclature? Explain with examples.
Asked by Shreyash.98rana | 19 Oct, 2014, 03:07: PM
Dear Shreyash.98rana@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Solution of your first query,
1-methyl propane is nothing but n-butane which is a structural isomer of C4H10 (butane).
The isomers of C4H10 (butane) are as follows:
n-butane and 2-methyl propane.
Regards
Topperlearning Team.
Topperlearning Team.
Answered by Prachi Sawant | 20 Oct, 2014, 10:56: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 11:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 09:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 09:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 08:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 10:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 03:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 05:52: PM
CBSE 10 - Chemistry
Asked by amitpass78 | 09 Jan, 2019, 10:35: PM