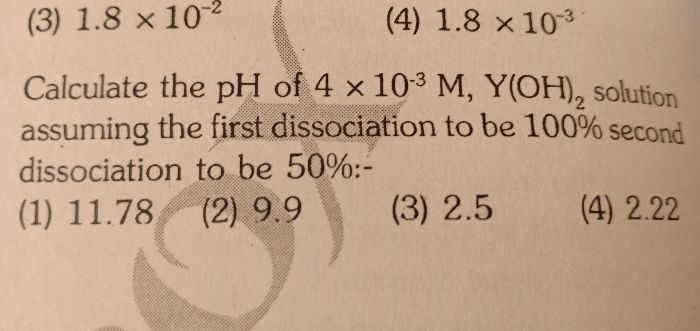CBSE Class 11-science Answered
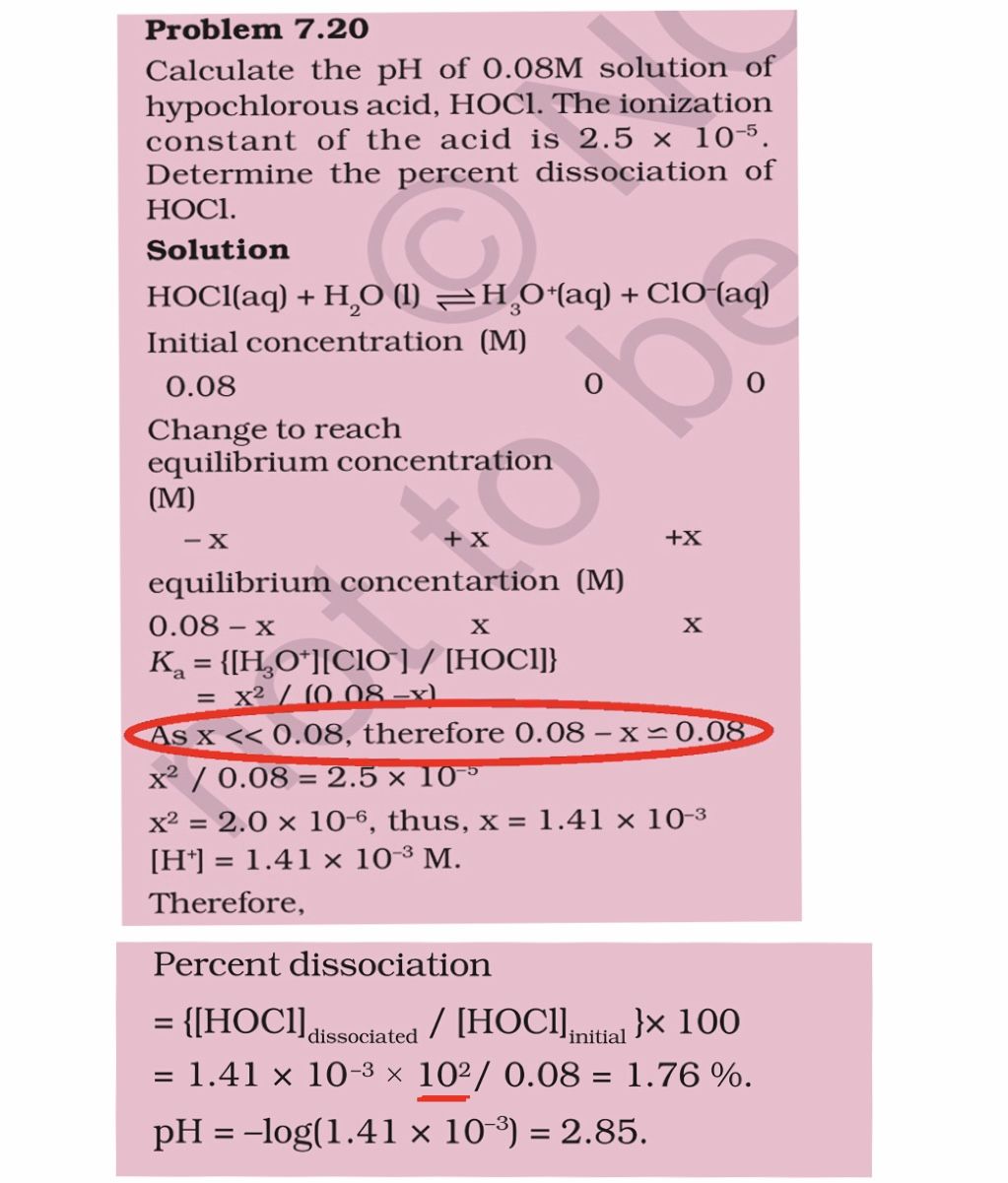
in this problem,, the answer seems to suggest that ‘x’ is an infinitesimally small change in the initial concentration of HOCL at equilibrium, rendering it’s concentration pretty much unchanged. i’ve been wondering as to how we know that ? ‘x’ being negligible i mean ?
i also do not understand why there is a random 10^2 being multiplied into the value of dissociated [HCOL] in the percent dissociation calculation. it seems to make no sense to me. unless i’m missing something.....
Asked by astutijoshi | 06 Jun, 2019, 07:09: PM
HOCl is a weak acid so when it is dissolved in water it's degree of dissociation is considered as very less as comopared to given concentration. because weak acids are not completely ionised. That's why x<<0.008
and 100 that is multplied for cnverting in percentage is written as 102.
Answered by Ravi | 07 Jun, 2019, 05:01: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by sarojlaxmiacharjya | 03 Jan, 2022, 08:50: PM
CBSE 11-science - Chemistry
Asked by cjam41665 | 09 Oct, 2021, 11:11: PM
CBSE 11-science - Chemistry
Asked by rishika62124 | 03 Mar, 2021, 05:02: AM
CBSE 11-science - Chemistry
Asked by jyotijhajharia39 | 06 Jan, 2021, 11:41: PM
CBSE 11-science - Chemistry
Asked by nsaikumar33 | 15 Aug, 2020, 11:50: AM
CBSE 11-science - Chemistry
Asked by swati2678 | 10 Aug, 2020, 01:58: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 17 Apr, 2020, 10:50: AM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 17 Apr, 2020, 10:44: AM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 14 Apr, 2020, 02:42: PM
CBSE 11-science - Chemistry
Asked by SanskarAgarwal86 | 29 Feb, 2020, 04:36: AM