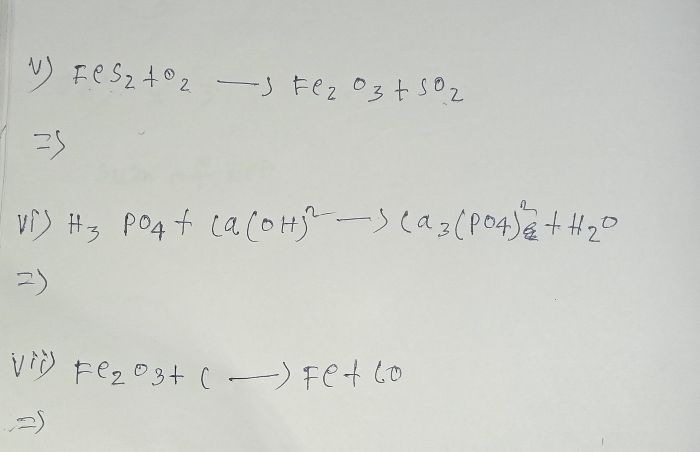CBSE Class 10 Answered
in lead nitrate why both sides brackets are used for the equation nitrate like pb(no3)2
Asked by ajayrath7 | 25 Mar, 2019, 10:08: AM
Nitrate is composed of group of atoms like one nitrogen atom and three oxygen atoms, 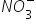
If nirate group is attached to an metal atom who has valency ore than 1 one should draw it in a bracket to avoid confusion of numbers.
NaNO3 in this compound sodium has valency one hence no need to write a bracket,
while in case of Magnesium nitrate, Mg(NO3)2 one should write nitrate group in bracket, to avoid the confusion.
Answered by Ramandeep | 25 Mar, 2019, 05:37: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by kashviS.shah | 14 Sep, 2022, 11:27: PM
CBSE 10 - Chemistry
Asked by saharupa28041 | 30 Jun, 2022, 11:29: PM
CBSE 10 - Chemistry
Asked by Sunita | 23 Feb, 2022, 06:25: PM
CBSE 10 - Chemistry
Asked by labheshvaidya | 11 Feb, 2022, 05:01: PM
CBSE 10 - Chemistry
Asked by sssaibadreeshwar | 27 Nov, 2021, 04:07: PM
CBSE 10 - Chemistry
Asked by tdeeksha3109 | 09 Nov, 2021, 02:11: PM
CBSE 10 - Chemistry
Asked by simratkaurr1 | 07 Jun, 2021, 12:55: PM