CBSE Class 12-science Answered
In groovs process zncl2 breaks the c-o then what is the meaning of it coordinate with the oxygen atom of the alcohol?
Asked by gargimoitreyee | 17 Mar, 2018, 04:46: PM
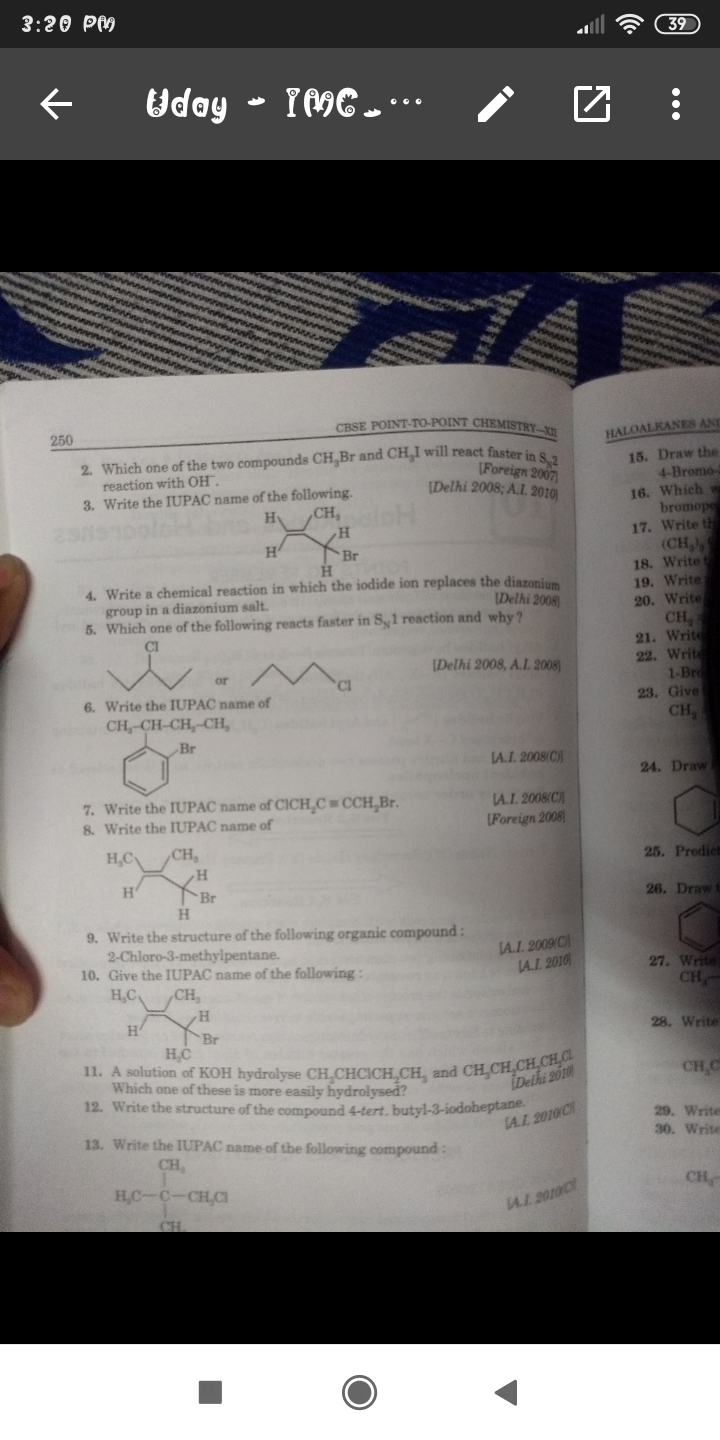
Grooves method, in this method with the help of 'Lucus reagent' i.e. HCl + ZnCl2 we prepared alkyl halide from alcohol,
As we know that in alcohols to replace OH group by Cl is very difficult that's why we used ZnCl2 here, Zn has more affinity towards the oxygen hence first it form a coordinate bond with oxygen of alcohol then it becomes -O+HZnCl and it is easier for chloride ion to replace this group and form alkyl chloride.
Answered by Ramandeep | 19 Mar, 2018, 09:13: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 01:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 05:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 04:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 10:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 01:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 02:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 08:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:34: PM




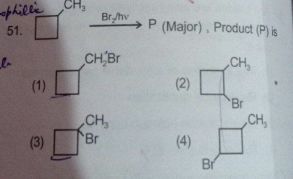
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-