ICSE Class 10 Answered
In alpha decay 2 protons and two neutrons are lost by an atom
Does the number of elcetrons reamin same
Asked by lovemaan5500 | 21 Dec, 2017, 04:36: PM
in alpha decay atomic number of alpha emiiting nucleus reduced by 2 and the alpha emitting nucleus is changed into different type of element.
For example in the following equation, Uranium is changed to thorium after alpha emission.
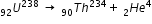
In this radioactive decay, thorium is neutral/excited atom but can have 90 electrons only, but the alpha particle is helium nucleus it does not have electrons. since Uranium is metal, the excess two electrons reside in the uranium metal as free eletrons and stay at the surface as surface charge
Answered by | 22 Dec, 2017, 12:35: PM
Concept Videos
ICSE 10 - Physics
Asked by anjukumarisah730 | 16 Mar, 2023, 10:30: PM
ICSE 10 - Physics
Asked by yashpatil4980.10sdatl | 11 Jun, 2020, 04:07: PM
ICSE 10 - Physics
Asked by arpitt682 | 17 Dec, 2019, 10:52: AM
ICSE 10 - Physics
Asked by pb_ckt | 04 Mar, 2019, 02:56: PM
ICSE 10 - Physics
Asked by chumki.banerjee001 | 03 Mar, 2019, 07:45: PM
ICSE 10 - Physics
Asked by DILIP | 06 Feb, 2019, 07:19: PM
ICSE 10 - Physics
Asked by pb_ckt | 05 Feb, 2019, 12:09: PM
ICSE 10 - Physics
Asked by pb_ckt | 05 Feb, 2019, 12:09: PM
ICSE 10 - Physics
Asked by gsitabhanu24 | 03 Feb, 2019, 10:25: AM
ICSE 10 - Physics
Asked by om.chaudhari1673 | 23 Jan, 2019, 04:48: PM



