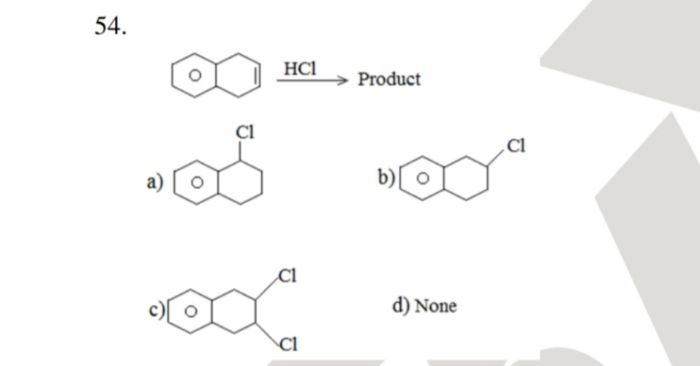
CBSE Class 12-science Answered
In 2nd plz explain how these two effects control orientation and reactivity
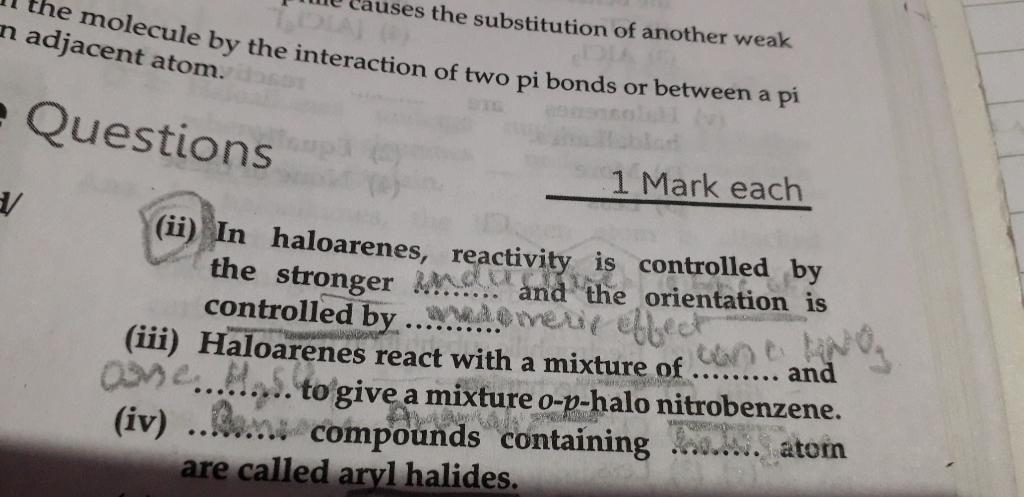
Asked by lovemaan5500 | 13 Jan, 2020, 09:36: PM
- Chlorine withdraws electrons through inductive effect and releases electrons through resonance.
- Through inductive effect, chlorine destabilises the intermediate carbocation formed during the electrophilic substitution.
- Through resonance, halogen tends to stabilise the carbocation and the effect is more pronounced at ortho- and para- positions.
- The inductive effect is stronger than resonance and causes net electron withdrawal and thus causes net deactivation.
- The resonance effect tends to oppose the inductive effect for the attack at ortho- and para positions and hence makes the deactivation less for ortho- and para attack.
- Reactivity is thus controlled by the stronger inductive effect and orientation is controlled by resonance effect.
Answered by Ramandeep | 15 Jan, 2020, 10:55: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by jaiadithya05 | 20 Sep, 2021, 07:20: PM
CBSE 12-science - Chemistry
Asked by shantasharma611 | 01 May, 2021, 01:51: PM
CBSE 12-science - Chemistry
Asked by sivaveeramachaneni9 | 08 Feb, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by kalkikai33 | 01 Jun, 2020, 03:40: PM
CBSE 12-science - Chemistry
Asked by subhasmitaswainstudent | 02 May, 2020, 01:22: PM
CBSE 12-science - Chemistry
Asked by buluacharya123 | 25 Apr, 2020, 11:37: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 10:58: PM
CBSE 12-science - Chemistry
Asked by tn6380313887.mohanviji | 09 Mar, 2020, 09:19: AM
CBSE 12-science - Chemistry
Asked by nidhi.jain0212 | 07 Mar, 2020, 01:30: PM