CBSE Class 11-science Answered

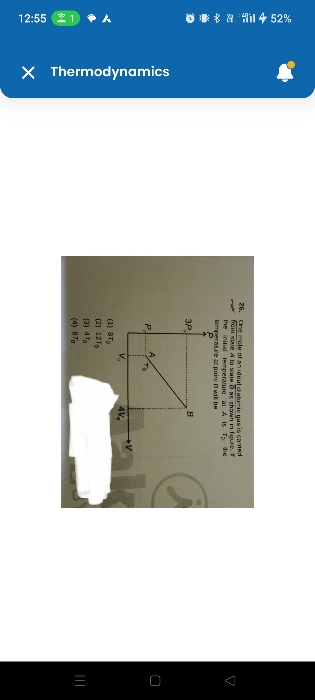
The complete process can be divided into two stages i.e.
Stage 1: when the work is done by the surrounding on the gas in compressing it at constant pressure.
Assuming the process to be adiabatic, i.e. q=0
According to first law of thermodynamics q = ΔU + W
Therefore, increase in internal energy = - work done by the gas
= - p×(V2-V1) = - 100×(1-2) = 100 joule
Stage2: when heat is supplied to the gas by keeping volume of gas constant (i.e. isochoric process)
Here change in internal energy is equal to heat supplied as work done by the gas is zero.
Therefore increase in internal energy = 150 joule.
The total increase in internal energy of gas = 100 + 150 = 250 joule.
* note: In the above question we have assumed the first process to be adiabatic as nothing is mentioned.