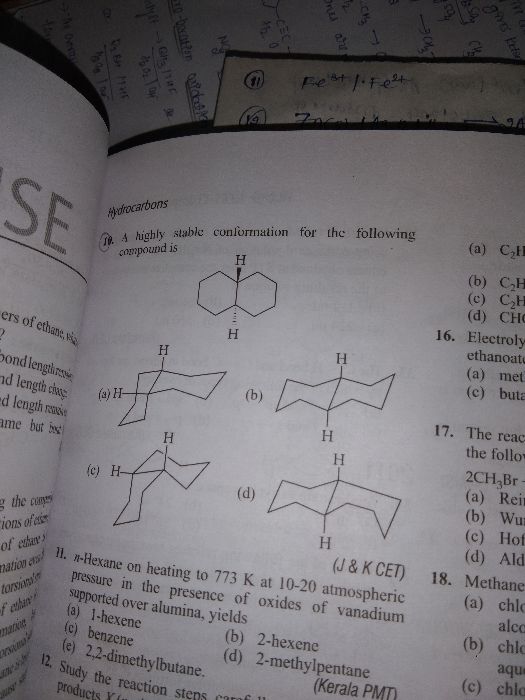
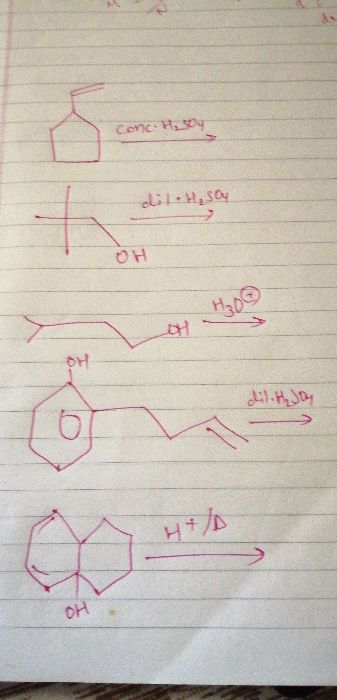
CBSE Class 11-science Answered
Asked by preethz chandra | 09 Apr, 2014, 11:17: PM
Consider an organic compound like phenol.So let us see the effect of substituents on the acidic character of pehnols.
The effect of substituents on the acidic character of phenols can be easily understood in terms of stability of phenoxide ion relative to phenol.
Any group that stabilises the phenoxide ion more than the acid would make the reaction more towards forward direction and would increase the acidity.
On the other hand, a group that makes the phenoxide ion less stable would decrease the acidity.
Alkyl group is an electron donating group and intensifies the negative charge and therefore destabilises the phenoxide ion w.r.t phenol. So the acidic strength decreases.
Answered by | 10 Apr, 2014, 05:36: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM