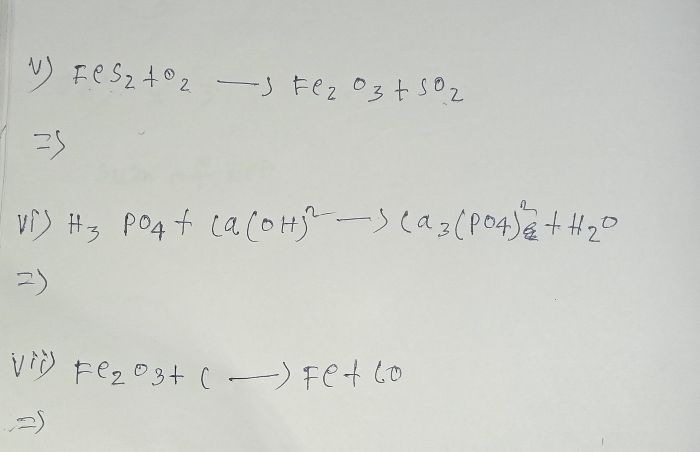CBSE Class 10 Answered
Question 2
Solution
A balanced chemical equation means that the number of atoms of each element are equal on both the sides. Thus, the law of conservation of mass hold true.
Asked by Madhuchhanda Chakraborty | 19 May, 2014, 03:11: PM
For a balanced chemical equation, the number of atoms of each element are equal on both sides. This is an accordance with the law of conservation of mass.
So the correct options are:
(A) Number of atoms of each element are equal on both the sides.
and
(D) Law of conservation of mass holds true.
Answered by Vaibhav Chavan | 20 May, 2014, 10:17: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by kashviS.shah | 14 Sep, 2022, 11:27: PM
CBSE 10 - Chemistry
Asked by saharupa28041 | 30 Jun, 2022, 11:29: PM
CBSE 10 - Chemistry
Asked by Sunita | 23 Feb, 2022, 06:25: PM
CBSE 10 - Chemistry
Asked by labheshvaidya | 11 Feb, 2022, 05:01: PM
CBSE 10 - Chemistry
Asked by sssaibadreeshwar | 27 Nov, 2021, 04:07: PM
CBSE 10 - Chemistry
Asked by tdeeksha3109 | 09 Nov, 2021, 02:11: PM
CBSE 10 - Chemistry
Asked by simratkaurr1 | 07 Jun, 2021, 12:55: PM