CBSE Class 11-science Answered
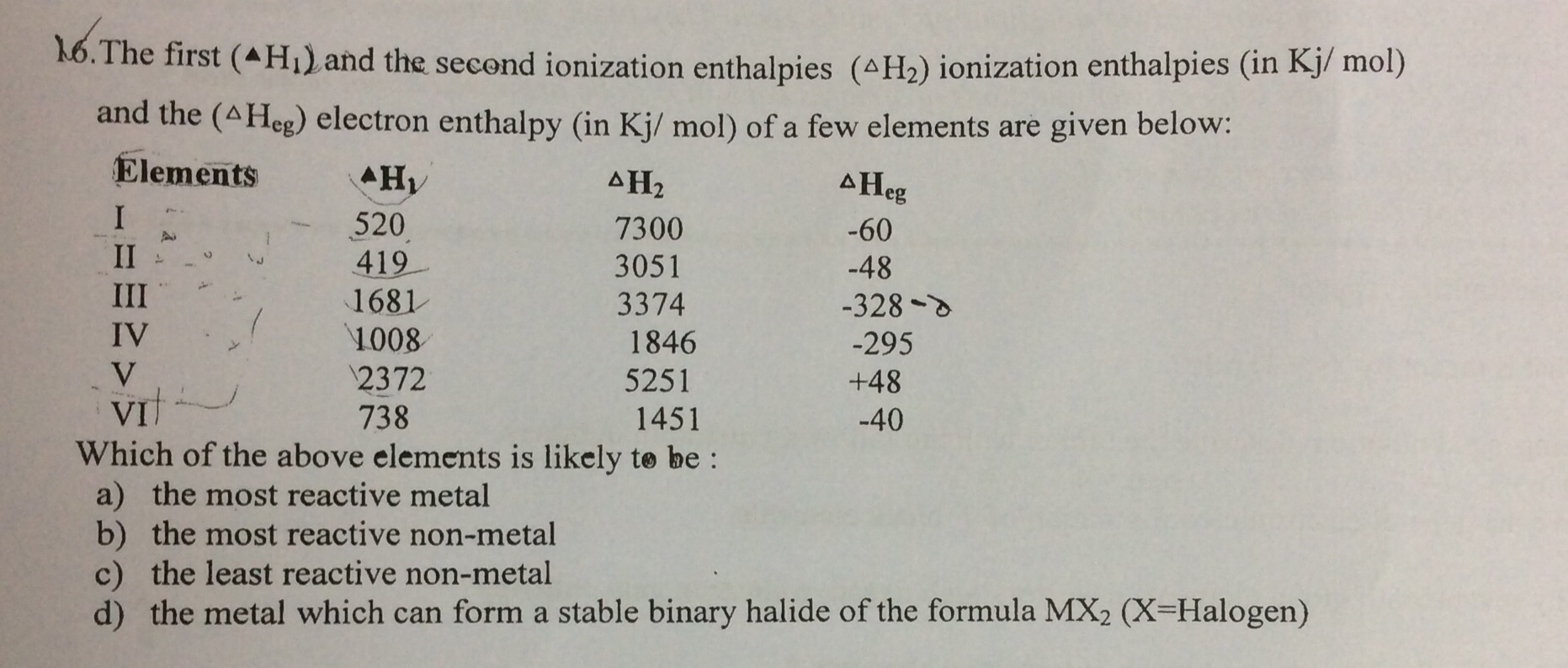
(a) Element II is likely to be the most reactive metal as it has the lowest first ionization enthalpy (ΔH1) and a low negative electron gain enthalpy (ΔHeg).
(b) Element III is likely to be the most reactive non–metal as it has a high first ionization enthalpy (ΔH1) and the highest negative electron gain enthalpy (ΔHeg).
(c) Element V is likely to be the least reactive non–metal since it has a very high first ionization enthalpy (ΔH2) and a positive electron gain enthalpy (ΔHeg).
(d) Element VI has a low negative electron gain enthalpy (ΔHeg). Thus, it is a metal. Further, it has the lowest second ionization enthalpy (ΔH2). Hence, it can form a stable binary halide of the formula MX2 (X=halogen).