JEE Class main Answered
If silver atom occupy at centre, gold occupy body centre and lattice is CCP constituted by copper the formula of alloy will be
Asked by inbasri224 | 23 Feb, 2019, 11:48: PM
Let us find the effective number of Cu, Ag and Au atoms in a unit cell,
1) The effective number of Cu atoms in a unit cell =
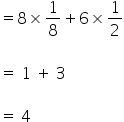
2) The effective number of Ag atoms in a unit cell =
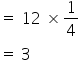
3) The effective number of Au atoms in a unit cell = 1
So, the general formula of the alloy is Cu4Ag3Au
Answered by Ramandeep | 25 Feb, 2019, 12:00: PM
JEE main - Chemistry
Asked by astutijoshi | 03 Jun, 2019, 05:12: PM
JEE main - Chemistry
Asked by inbasri224 | 23 Feb, 2019, 11:48: PM
JEE main - Chemistry
Asked by Jagilinkinylu | 29 Dec, 2018, 08:03: PM

