CBSE Class 11-science Answered
if Qc is greater than Kc.reaction goes backward.and vice-versa.
HOW does that work?(just to remember this relation,im asking)
Asked by vicky Sekaran | 12 Nov, 2014, 10:39: PM
If Qc > Kc , then the reaction favors the reactants. This means that in the equation 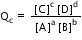 , the ratio of the numerator (the concentration or pressure of the products) to the denominator (the concentration or pressure of the reactants) is larger than that for Kc indicating that more products are present than there would be at equilibrium. Because reactions always tend toward equilibrium (Le Chatelier's principle), the reaction produces more reactants from the excess products, therefore causing the system to shift to the LEFT. This allows the system to reach equilibrium.
, the ratio of the numerator (the concentration or pressure of the products) to the denominator (the concentration or pressure of the reactants) is larger than that for Kc indicating that more products are present than there would be at equilibrium. Because reactions always tend toward equilibrium (Le Chatelier's principle), the reaction produces more reactants from the excess products, therefore causing the system to shift to the LEFT. This allows the system to reach equilibrium.
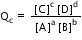 , the ratio of the numerator (the concentration or pressure of the products) to the denominator (the concentration or pressure of the reactants) is larger than that for Kc indicating that more products are present than there would be at equilibrium. Because reactions always tend toward equilibrium (Le Chatelier's principle), the reaction produces more reactants from the excess products, therefore causing the system to shift to the LEFT. This allows the system to reach equilibrium.
, the ratio of the numerator (the concentration or pressure of the products) to the denominator (the concentration or pressure of the reactants) is larger than that for Kc indicating that more products are present than there would be at equilibrium. Because reactions always tend toward equilibrium (Le Chatelier's principle), the reaction produces more reactants from the excess products, therefore causing the system to shift to the LEFT. This allows the system to reach equilibrium.
Answered by Arvind Diwale | 13 Nov, 2014, 11:52: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by visank90 | 24 Nov, 2023, 10:45: AM
CBSE 11-science - Chemistry
Asked by rakhikumarithakur4 | 22 May, 2020, 08:21: PM
CBSE 11-science - Chemistry
Asked by arshrana3272 | 04 Mar, 2020, 02:57: PM
CBSE 11-science - Chemistry
Asked by hsdhall.2005 | 12 Nov, 2019, 11:40: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Jul, 2018, 08:52: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 05:17: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 05:17: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 03:08: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 04:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 04:15: PM




 6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added
6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added