CBSE Class 12-science Answered
If meta position shows no mesomerism effect then how come -R group is meta ditective?
Asked by peedyojb | 24 Oct, 2017, 04:54: PM
The direction of substitution is depend on -R group which is present on benzene ring, thus there are two types of substituents 1) ortho & para directing 2) meta directing.
for example the electrophlic substitution of nitro group to the nitro benzene

in above structeres the positive charge is at the ortho and para positions. therefore they are unreactive to the attacking electrophile which is also positively charged (NO2+), naturally now meta position becomes electron rich than ortho and para positions therefore in this way without showing resonance effect at meta position meta substitution takes place
Answered by Ramandeep | 25 Oct, 2017, 11:11: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 29 Jun, 2021, 08:36: AM
CBSE 12-science - Chemistry
Asked by mahaynoorf | 17 Oct, 2020, 08:39: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 10:58: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 11:54: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 28 Aug, 2019, 07:45: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 20 May, 2019, 11:46: PM
CBSE 12-science - Chemistry
Asked by shobhit | 21 Feb, 2019, 11:01: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:43: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:41: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Apr, 2014, 08:33: AM




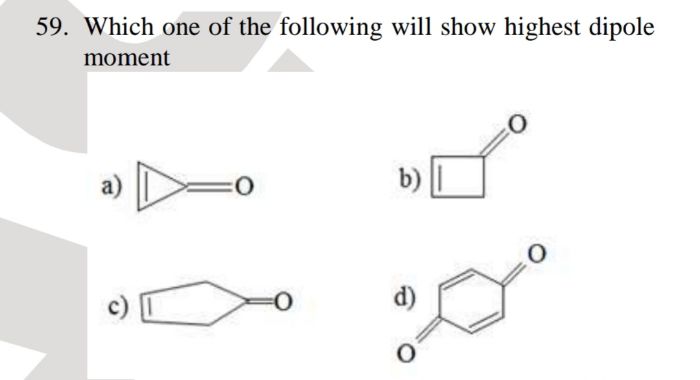
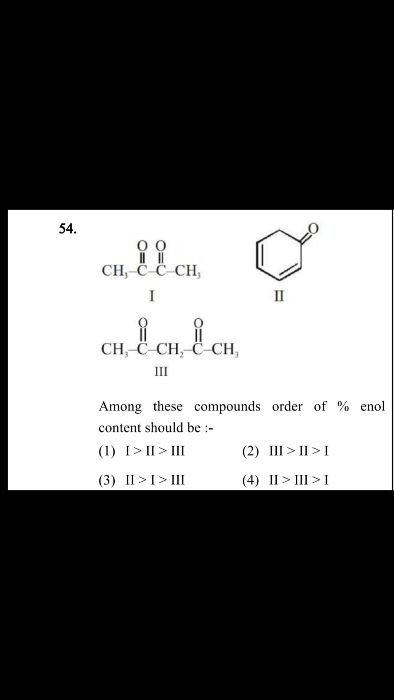
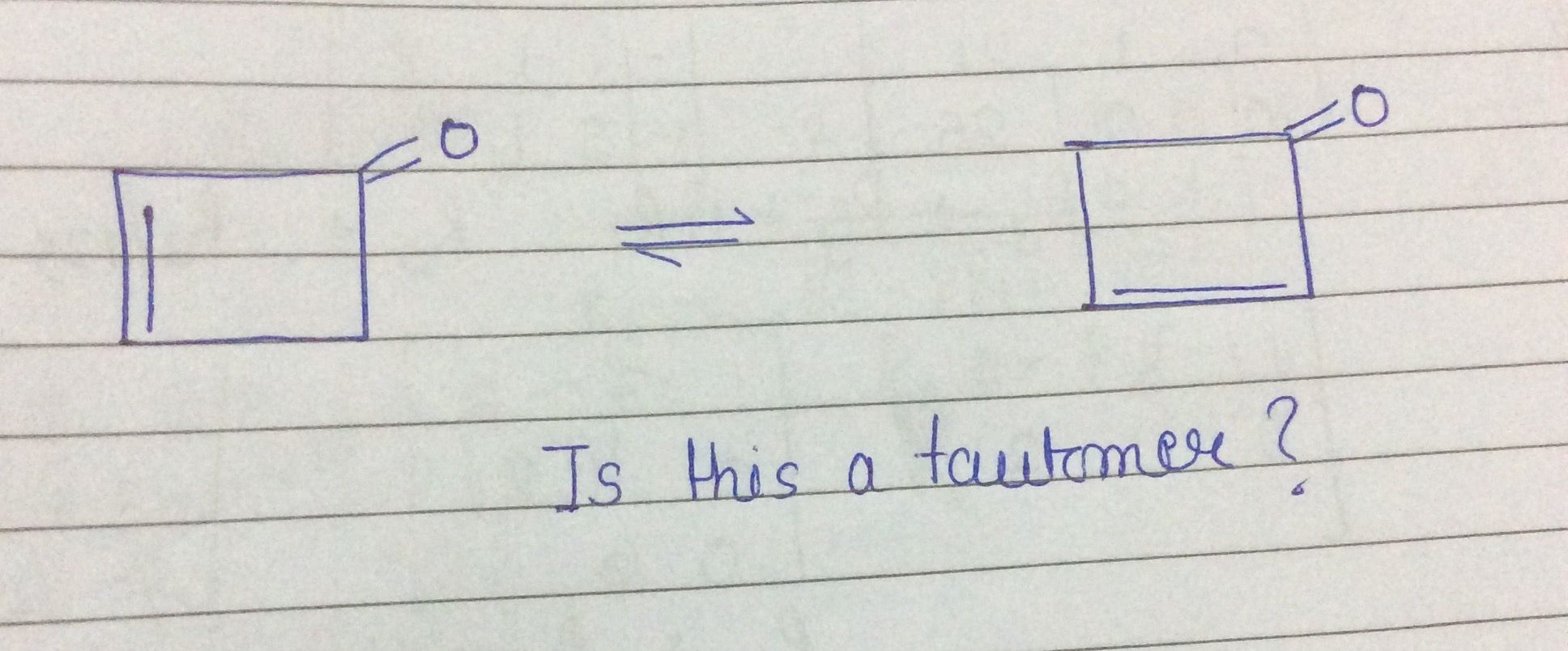
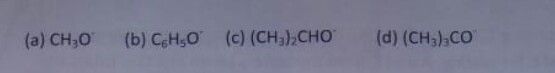
 Y reacts with Z to give
(1)
Y reacts with Z to give
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)

 Z is :-
(1)
Z is :-
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)

