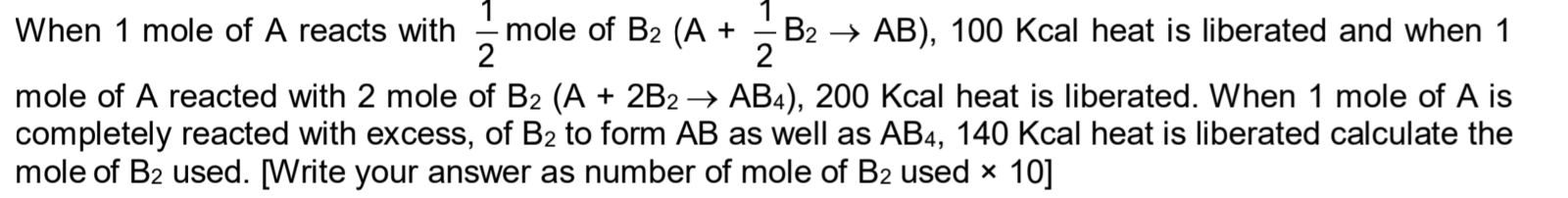CBSE Class 11-science Answered
If isobutane and n-butane are present in a gas, then how much oxygen should be required for complete combustion of 10 kg of this gas
Asked by Anil | 16 May, 2017, 03:36: PM
If isobutane and n-butane are present in a gas, then how much oxygen should be required for complete combustion of 10 kg of this gas:
Isobutane and n-Butane have same chemical formula (C4H10) and give same combustion reaction.
2C4H10+13O2→8CO2+10H2O
This means 2 moles of this mixture requires 13 moles of oxygen.
Molecular mass of butane=58g/mol
Molecular mass of oxygen=32g/mol
Thus, 116 g mixture needs 13 x 32 g = 416 g oxygen
10 kg of the mixture = 10 x 103 g needs = 416 x 10 x 103 / 116 = 35862.06 g of oxygen
Therefore, 35.862 kg oxygen is used.
Answered by Prachi Sawant | 16 May, 2017, 04:53: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 10:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 03:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 10:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 03:58: PM
CBSE 11-science - Chemistry
Asked by pb_ckt | 19 May, 2019, 11:56: PM