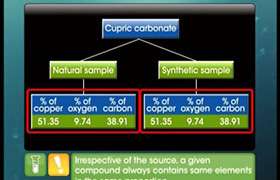
CBSE Class 11-science Answered
If a certain oxide of nitrogen weighing 0.11 g gives 56ml of nitrogen and another oxide of nitrogen weighing 0.15 g gives the same volume of nitrogen (bothe at STP) , show that these results support the law of multiple proportions.
Asked by Balbir | 09 May, 2016, 09:18: PM
Answered by Prachi Sawant | 10 May, 2016, 11:40: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by dhrubapratimc | 13 Sep, 2023, 09:34: PM
CBSE 11-science - Chemistry
Asked by abhiabhishek842006 | 09 Jan, 2023, 08:34: PM
CBSE 11-science - Chemistry
Asked by Shashisinghkusum | 13 Jul, 2022, 02:44: PM
CBSE 11-science - Chemistry
Asked by shivanshiarora3457 | 09 Jun, 2022, 03:28: PM
CBSE 11-science - Chemistry
Asked by rishamariyam222 | 28 Sep, 2021, 09:18: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 08 Feb, 2021, 10:52: AM
CBSE 11-science - Chemistry
Asked by manteaditya8 | 08 Jan, 2021, 01:45: PM
CBSE 11-science - Chemistry
Asked by muanputhomte4 | 11 Nov, 2020, 09:01: PM
CBSE 11-science - Chemistry
Asked by pradeepprince858 | 07 Aug, 2020, 12:54: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 28 Jul, 2020, 11:03: AM