CBSE Class 9 Answered
Ice at 0 Celsius appears cooler than Water at 0 Celsius. Why?
Asked by prakash.sanyasi | 29 Jul, 2019, 10:58: PM
Due to the more latent heat absorbed by ice as compare to water at same temperature, ice appears more colder than water.
The amount of heat energy required to by ice to change into water is latent heat of fusion and its value is 80 cal/g.
The specific heat capacity of water is 1 cal/gm per °C.
So ice absorbs more energy to increase its temperature than water at 0 °C.
Answered by Varsha | 30 Jul, 2019, 10:31: AM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rubinapathan228 | 29 Jun, 2023, 05:45: PM
CBSE 9 - Chemistry
Asked by yforyt3672 | 09 Apr, 2023, 07:37: PM
CBSE 9 - Chemistry
Asked by pranavtamboli65.9 | 02 Aug, 2022, 08:12: PM
CBSE 9 - Chemistry
Asked by ritapriya126 | 20 Apr, 2022, 04:01: PM
CBSE 9 - Chemistry
Asked by renukaramaswamyr | 08 Sep, 2021, 12:32: PM
CBSE 9 - Chemistry
Asked by gpranithasri | 27 Jul, 2021, 01:23: PM
CBSE 9 - Chemistry
Asked by suhanivasi007 | 04 May, 2021, 05:52: PM
CBSE 9 - Chemistry
Asked by pk36229905 | 06 Apr, 2021, 07:38: AM
CBSE 9 - Chemistry
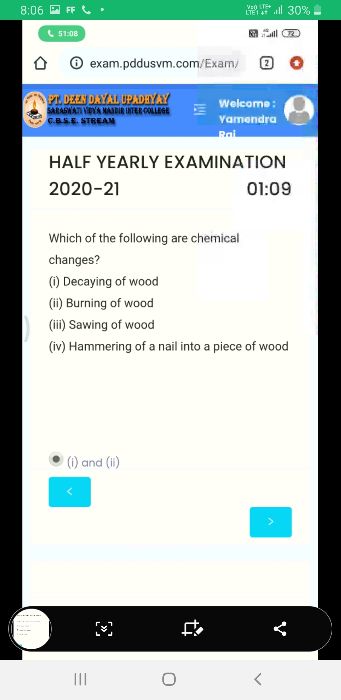
Asked by anubhavev | 30 Oct, 2020, 06:35: PM