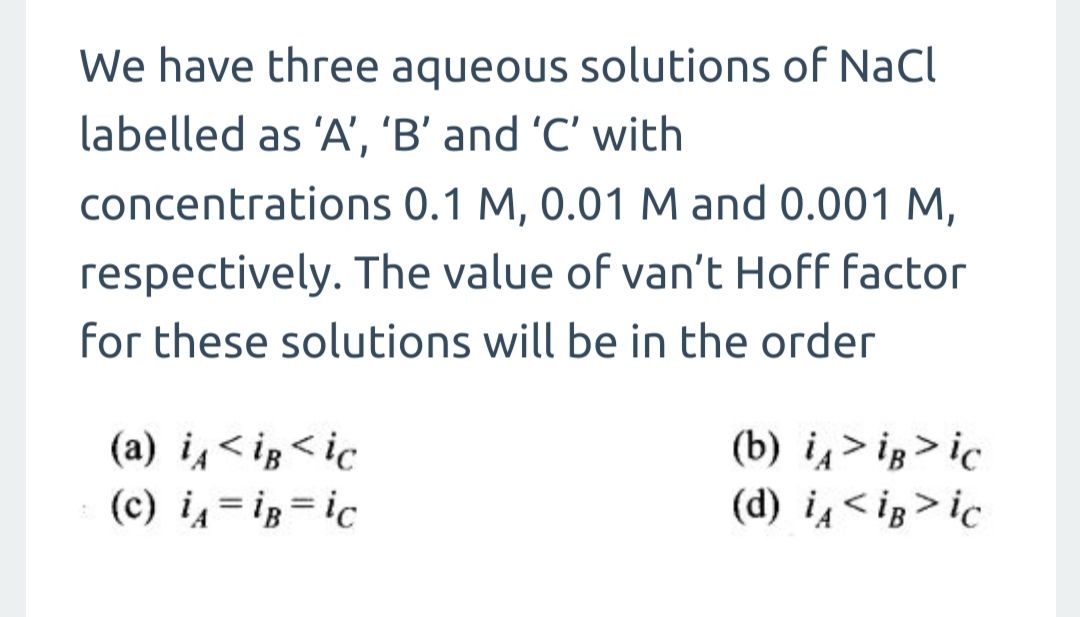
CBSE Class 12-science Answered
I got a project on chemistry where I have to do an experiment at home. Please suggest me a topic keeping in mind that the required materials can be easily found that can be done at home. Please dont confuse with the name of the chapter and the topic name that Ihave given. It was compulsory to fill those up.
Asked by Bohniman Bhuyan | 12 Nov, 2015, 12:44: PM
Dear bohnimanbhuyan@yahoo.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
The scope of this helpdesk is to answer subject related queries which are relevant to understanding concepts and solving problems. Hence we regret that we would not be able to answer your query.
Regards
Topperlearning Team.
Answered by Vaibhav Chavan | 12 Nov, 2015, 02:17: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 08:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 04:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 10:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 03:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM