ICSE Class 9 Answered
Hydrogen can be prepared with the metal zinc by using a.acid b.alkali c.water
Asked by nareshkumar5862 | 10 Oct, 2018, 08:37: PM
Hydrogen can be prepared with the metal zinc by using acid, alkali and water in the form of steam.
a) Acid:
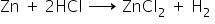
b) Alkali:

c) Water in the form of steam:
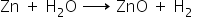
Answered by Ramandeep | 11 Oct, 2018, 10:07: AM
Concept Videos
ICSE 9 - Chemistry
Asked by enakshipal07 | 19 Nov, 2023, 11:45: AM
ICSE 9 - Chemistry
Asked by palrinabiwas | 22 Nov, 2021, 08:39: AM
ICSE 9 - Chemistry
Asked by amdeekshith48 | 20 Feb, 2021, 01:37: PM
ICSE 9 - Chemistry
Asked by nilimamarak25986 | 27 Apr, 2020, 04:23: PM
ICSE 9 - Chemistry
Asked by arunkumar9580616109 | 22 Feb, 2020, 07:05: PM
ICSE 9 - Chemistry
Asked by prithvigupta | 02 Feb, 2020, 06:26: PM
ICSE 9 - Chemistry
Asked by qurikriti | 22 Oct, 2019, 07:48: PM
ICSE 9 - Chemistry
Asked by qurikriti | 22 Oct, 2019, 07:46: PM
ICSE 9 - Chemistry
Asked by jhajuhi19 | 13 Jun, 2019, 07:56: PM
ICSE 9 - Chemistry
Asked by nareshkumar5862 | 10 Oct, 2018, 08:37: PM







