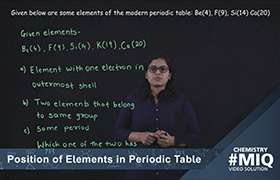
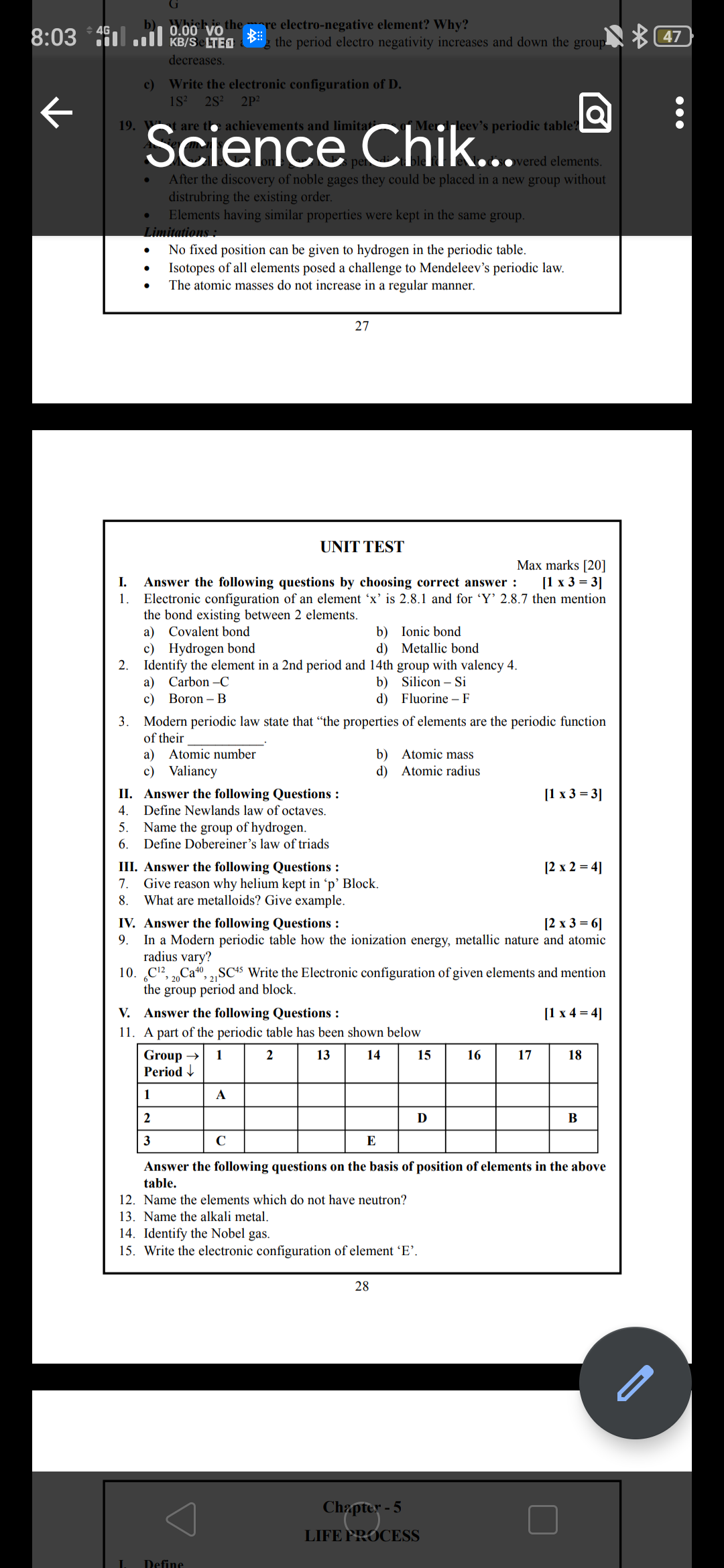
CBSE Class 10 Answered
How were Mendeleev's limitations in his periodic table rectified in the modern periodic table?
Asked by Tarun Rath | 24 Jan, 2014, 05:00: PM
Hi Biplav,
- In Mendeleev’s periodic table, the noble elements were not included in the table as they were not discovered at that time.
In Modern periodic table, the noble elements were placed in group 18 as a last and separated group.
- In Mendeleev’s periodic table, elements with larger atomic weights were placed before elements with smaller atomic weight without any justified reason.
In modern periodic table, all were arranged systematically with increasing atomic number and weight too.
- In Mendeleev’s periodic table, some different elements were grouped together while some similar elements were placed in different groups. Ex: alkali metals like Li , Na, K were placed with coinage metals Cu, Ag, Au etc.
In Modern periodic table there was no such confusion, the coinage metals have their own position in d-block and alkali metals are placed in s-block elements.
- In Mendeleev’s periodic table, the isotopes were placed in different positions.
In Modern periodic table, the isotopes were assigned same position as they have same atomic number.
- In Mendeleev’s periodic table, elements namely Fe, Co, Ni were placed together in Group VIII.
In Modern periodic table, these elements were placed under d-block elements in different groups.
Answered by Karishma Kapoor | 24 Jan, 2014, 06:34: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM