CBSE Class 12-science Answered
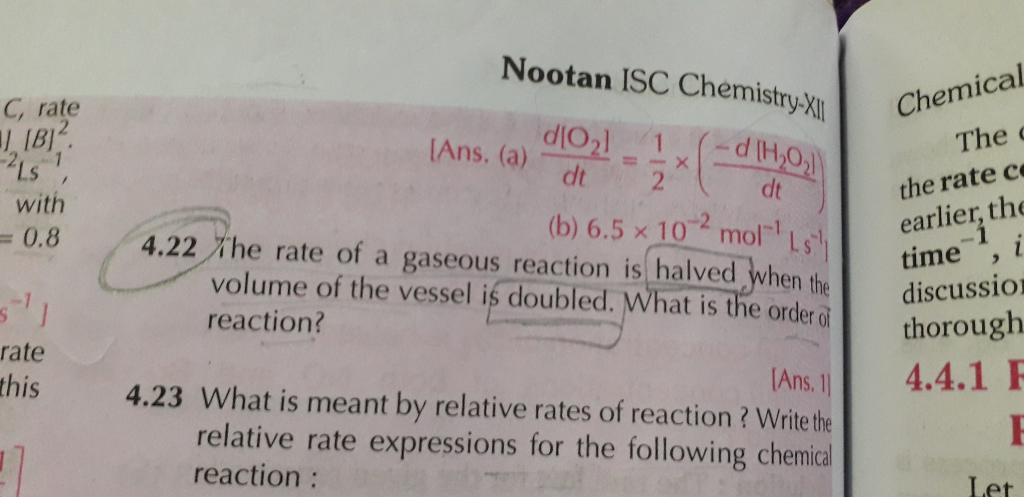
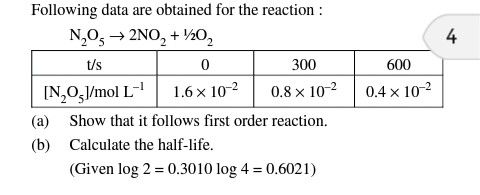
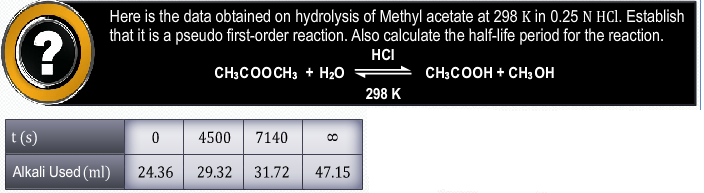
How we get to know that 2 moles of NH3 gives out N2 and 3 moles of H2 in the presence of platinum is a zero order even if the 2 moles are present which gives the sum of power 2 as per the definition of order of the reaction but how it is zero and as well as how it is not depending on the concentration of the reactants ?
Asked by deepu | 13 Sep, 2014, 12:24: AM
Dear dubey.bharat7@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
The order of a reaction cannot be deduced from the chemical equation of the reaction. Reactions in heterogenous system usually follow zero order kinetics. In such a system the reactants are adsorbed on the surface of a solid catalyst. At low concentrations of the reactant, the rate of the reaction depends on the fraction of the surface covered by the reactant. However, at higher concentrations the surface of the catalyst becomes fully covered and any further increase in the concentration of the reactants does not affect the rate of the reaction.
So depending upon concentration we can come to know whether the reaction is first order or zero order. With the help of stoichiometriy we can come to know the exact number of moles produced by NH3.Regards
Topperlearning Team.
Answered by Arvind Diwale | 23 Sep, 2014, 12:01: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 07:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 03:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 10:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 02:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 08:27: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 01:37: PM