CBSE Class 11-science Answered
How to prove the conditions for a real gas to be ideal?
Asked by tinku4121999 | 17 Sep, 2015, 08:03: PM
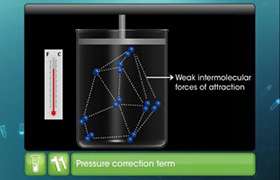
- Gases show deviation from ideal behaviour because of two faulty assumptions:
- There is no force of attraction between the molecules of a gas.
- Volume of the molecules of a gas is negligibly small in comparison to the space occupied by the gas.
- At low temperature and high pressure, gases deviate from ideal behaviour, i.e. gases behave as real gases.
- At low pressure and high temperature gases show ideal behaviour, i.e. gases behave as ideal gases.
- Plot of pressure–volume (pV) versus pressure (p) at constant temperature for ideal and real gases.
- Plot of pressure (p) versus volume (V) for ideal and real gases.
- Van der Waals equation of state is given below:
- The deviation from ideal behaviour can be measured in terms of the compressibility factor Z, which is the ratio of product pV and nRT.
- The deviations from ideal behavior become less and less with increase in temperature.
- The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure is called Boyle temperature or Boyle point.
Answered by Prachi Sawant | 18 Sep, 2015, 09:27: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 26 Jan, 2019, 12:57: PM
CBSE 11-science - Chemistry
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:19: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM