CBSE Class 12-science Answered
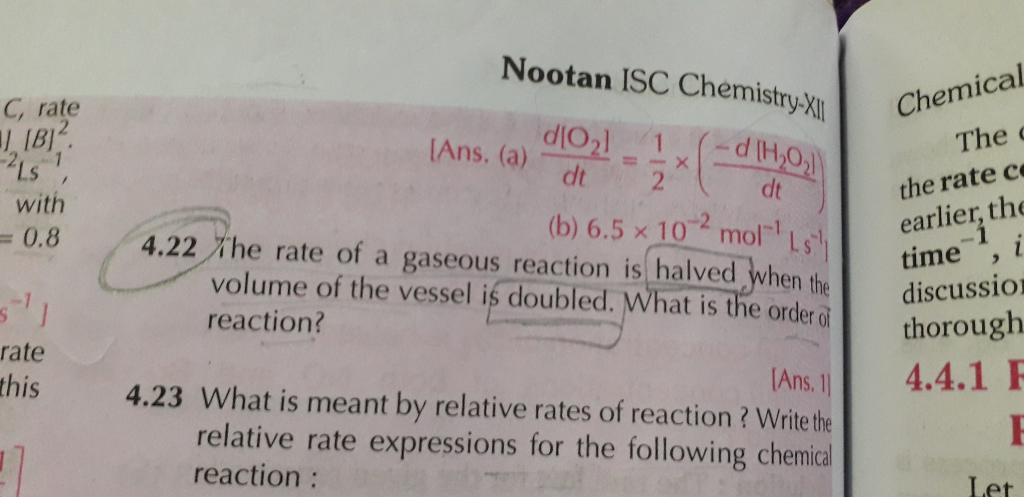
how to identify the orders of any reaction?
Asked by vasturushi | 06 Dec, 2017, 02:40: PM
In the rate equation
Rate = K[A]x [B]y
The x and y represents the dependence of the rate of reaction to change in the concentration of Aand B.
The sum of these exponents (x + y) in the above equation gives the overallorder of a reaction.
Hence,
The sum of powers of the concentration of the reactants in the rate law expression is called the order of that chemical reaction.
For example, if the rate expression of a particular reaction is
Rate = K[A]1/2 [B]3/2
Then the order of the reaction will be

The reaction is of second order.
Answered by Varsha | 06 Dec, 2017, 03:52: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 07:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 03:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 10:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 02:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 08:27: PM
CBSE 12-science - Chemistry
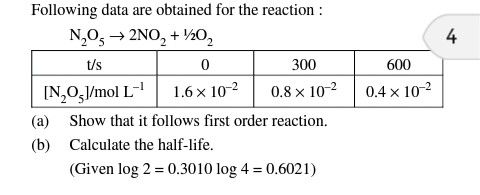
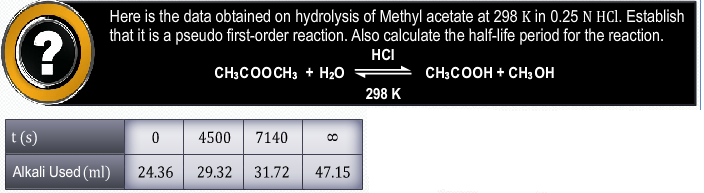
Asked by Topperlearning User | 27 Mar, 2014, 01:37: PM