CBSE Class 12-science Answered
HOW TO FIND VANT OF FACTOR
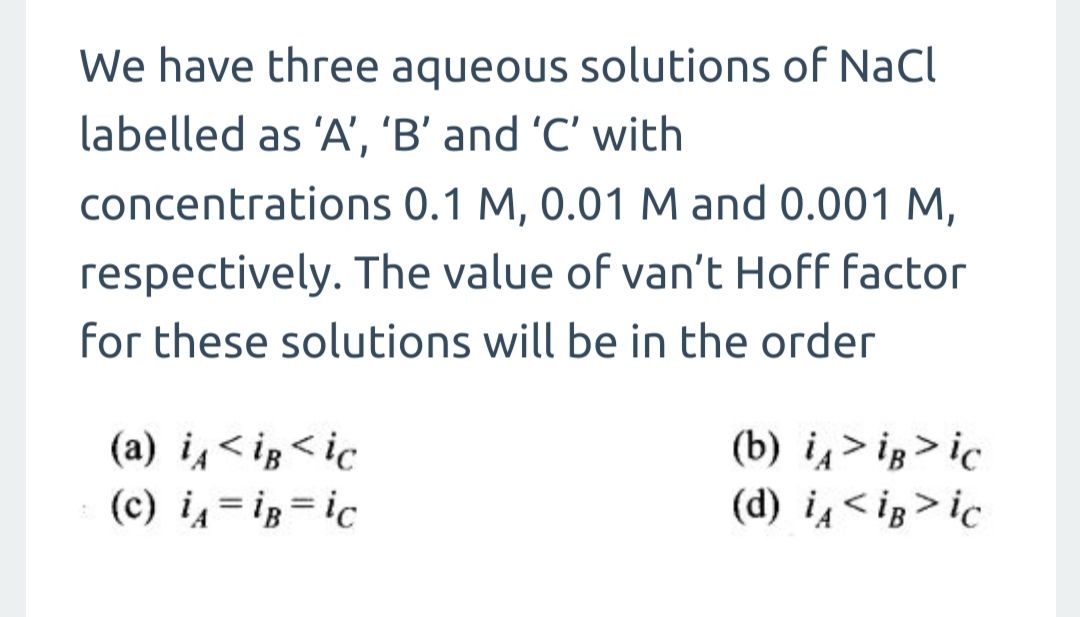
Asked by RAJAGUPTA | 01 Jan, 2020, 08:19: PM
The relationship between the actual number of moles of solute added to form a solution and the apparent number as determined by colligative properties is called the van't Hoff factor (i).
It is used to express the extent of association or dissociation of solute. If association or dissociation of solute particles takes place in the solution, then
Answered by Ramandeep | 02 Jan, 2020, 12:02: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 08:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 04:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 10:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 03:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM