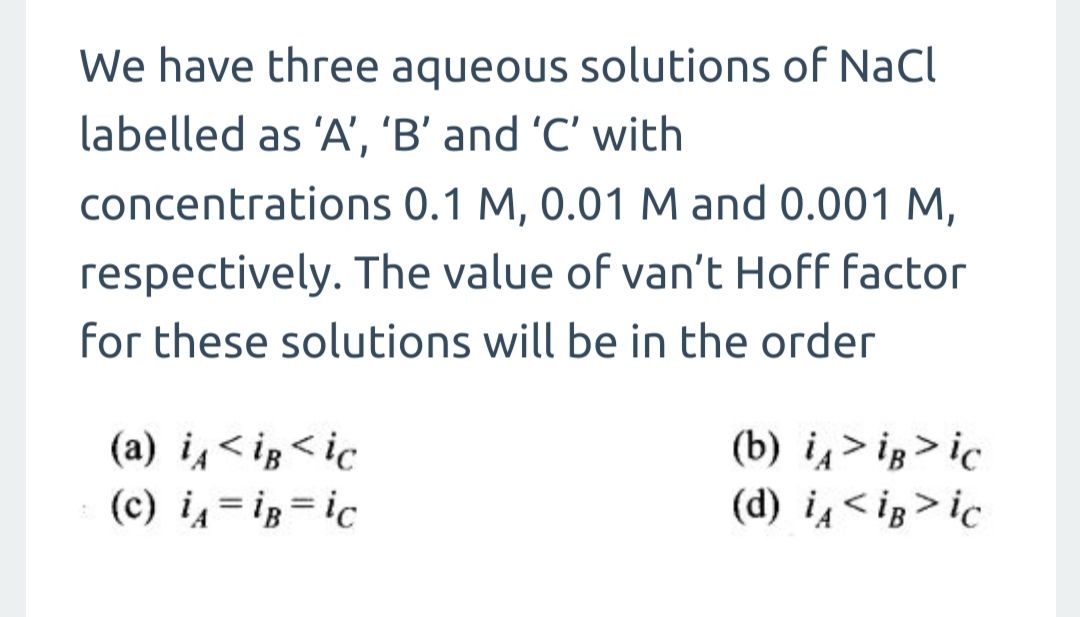
CBSE Class 12-science Answered
How to find out when to use 1-alpha is approximately 1 in the Ka equation? Also what is Solubility product, explain clearly.
Asked by Ravi Kumar | 02 Aug, 2014, 04:26: PM
Dear ravkumarc@yahoo.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Solution for your first query:
In Ka equation 1-alpha is approximately 1 when the change in the concentration is negligible. For example, if very minute quantity of solute such as 0.02 moles dissolved in 100 mL water then the change in concentration of water is negligible. Hence for water 1-alpha is approximatelt equal to 1.
Regards
Topperlearning Team.
Topperlearning Team.
Answered by Prachi Sawant | 05 Aug, 2014, 11:43: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 08:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 04:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 10:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 03:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM