CBSE Class 11-science Answered
How to count oxidation number?
Asked by AdityaNanda | 31 May, 2008, 10:14: PM
Calculating Oxidation Number or State :
This allows for a more formal, quantitative decription of the oxidation state and is based on looking at the relative electronegativity of the atoms attached to the atom being assessed. The algebraic sum of the oxidation states must equal the charge of the molecule.
If we are looking at a central carbon atom:
- for attached C atoms, i.e. C-C bonds electrons shared, count 0
- for attached X atoms, i.e. C-X bonds (X more electronegative), count -1 (per bond)
- for attached H atoms, i.e. C-H bonds (H is less electronegative than C), count +1
- Add the total for atoms attached to the C in question, then switch the sign.
If we are looking at a generic central atom
- for attached atom with the same electronegativity, i.e. bonding electrons shared, count 0
- for attached X atoms, i.e. X more electronegative, count -1 (per bond)
- for attached Y atoms, i.e. Y is less electronegative, count +1(per bond)
- Add the total for atoms attached to the central atom question, then switch the sign.
- Some examples are shown below:
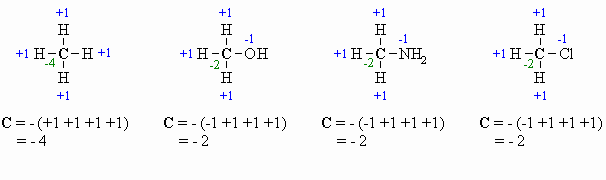
Answered by | 15 Jun, 2008, 11:11: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM






