CBSE Class 12-science Answered
How to compare the nuclear density of different elements if mass number and atomic number are given?
Asked by poojakamde3 | 08 Oct, 2017, 11:04: AM
- For finding density we need mass and volume of nucleus.
- Mass number is given as 55.85u (this is mass number of iron nucleus) here "u" is atomic mass unit. And 1u = 1.660539 x 10-27 Kg .
- Multiply 55.85 and 1.660539 x 10-27 you will get mass of iron nucleus in Kg.
- Volume of sphere (nucleus is sphere) is given by
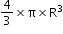 .Here,
.Here, 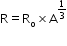 ,
, 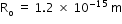 and A is atomic number.
and A is atomic number. - Find the volume and use the formula for density as
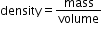 .
. - Use same method for nucleus of any other element and see which nucleus have more density and which ones less. In this way you can compare nuclear density of different elements.
Answered by Abhijeet Mishra | 08 Dec, 2017, 06:13: PM
Concept Videos
CBSE 12-science - Physics
Asked by rohandhawaniya17112006 | 06 Mar, 2024, 03:32: PM
CBSE 12-science - Physics
Asked by akashjyani705 | 06 Mar, 2022, 04:39: PM
CBSE 12-science - Physics
Asked by sharonashoksp | 27 Jun, 2021, 02:44: PM
CBSE 12-science - Physics
Asked by gaurish6247 | 07 Apr, 2021, 05:16: PM
CBSE 12-science - Physics
Asked by prerna.naga | 09 May, 2019, 08:51: AM
CBSE 12-science - Physics
Asked by kumarisakshi0209 | 17 Mar, 2019, 02:54: PM
CBSE 12-science - Physics
Asked by Amandeepsinghbedi26 | 26 Sep, 2018, 01:52: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 28 May, 2015, 09:51: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 28 May, 2015, 09:53: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM