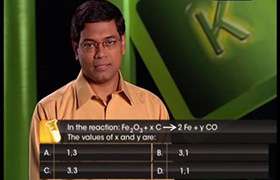CBSE Class 11-science Answered
how to calculate the oxidation number of Fe and S in compound FeS2 ? please explain briefly. i am in great trouble
Asked by ppratim02 | 29 Apr, 2015, 11:05: AM
The iron and sulfur atoms in FeS2 are arranged as Fe–S–S rather than S–Fe–S. 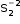 is the persulfide. In FeS2, iron is assigned an oxidation number of 2+, since persulfide has an oxidation number of −2, where each sulfur atom is assigned an oxidation number of -1.
is the persulfide. In FeS2, iron is assigned an oxidation number of 2+, since persulfide has an oxidation number of −2, where each sulfur atom is assigned an oxidation number of -1.
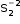 is the persulfide. In FeS2, iron is assigned an oxidation number of 2+, since persulfide has an oxidation number of −2, where each sulfur atom is assigned an oxidation number of -1.
is the persulfide. In FeS2, iron is assigned an oxidation number of 2+, since persulfide has an oxidation number of −2, where each sulfur atom is assigned an oxidation number of -1.
Answered by Arvind Diwale | 30 Apr, 2015, 08:31: AM
Concept Videos
CBSE 11-science - Chemistry
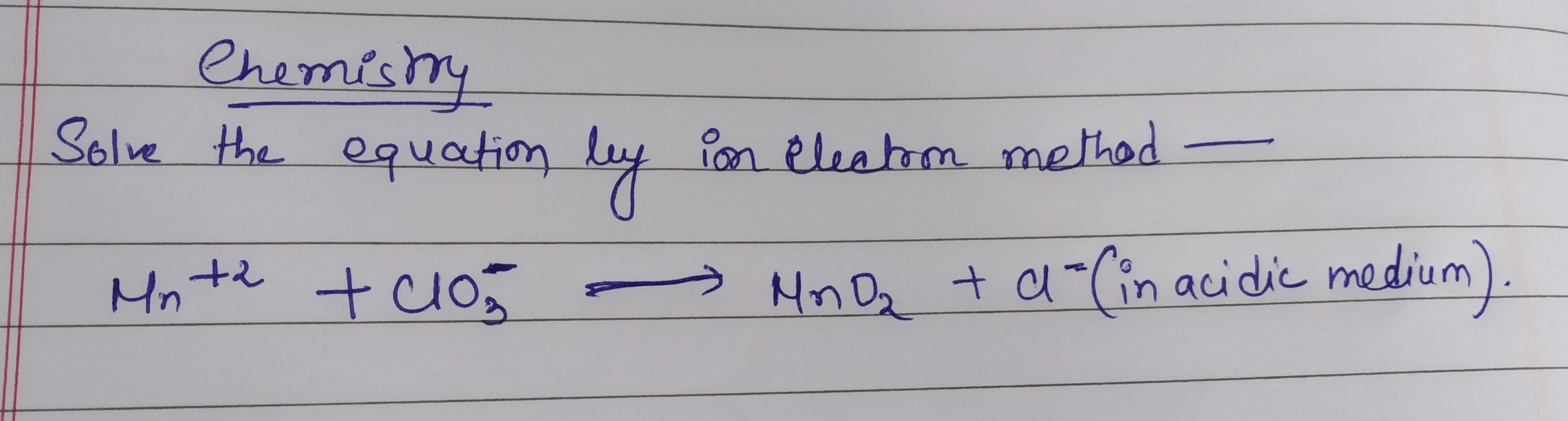
Asked by indranibaudya | 03 Oct, 2020, 08:12: PM
CBSE 11-science - Chemistry
Asked by Punshibakhuraijam2015 | 22 Sep, 2019, 08:40: PM
CBSE 11-science - Chemistry
Asked by kkdmmdsd | 09 Jun, 2019, 04:27: PM
CBSE 11-science - Chemistry
Asked by dheerajmathpal374 | 15 Sep, 2018, 10:43: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Aug, 2018, 06:29: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 18 Aug, 2018, 05:08: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 14 Aug, 2018, 06:06: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 10:29: PM
CBSE 11-science - Chemistry
Asked by g_archanasharma | 22 Mar, 2018, 10:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM