CBSE Class 11-science Answered
how is the radius of Bohr's orbit (r) is related to its energy level in hydrogen atom and hydrogen like atoms?
Asked by prema chauhan | 14 May, 2012, 10:52: PM
The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy: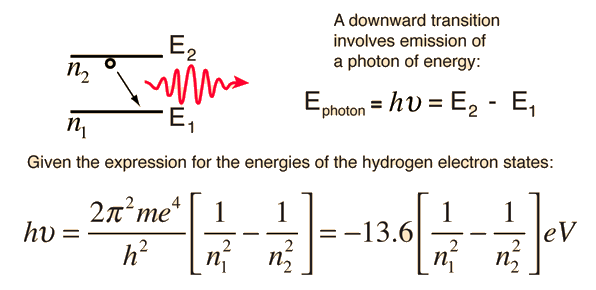
This is often expressed in terms of the inverse wavelength or "wave number" as follows:
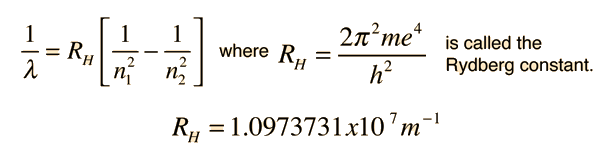
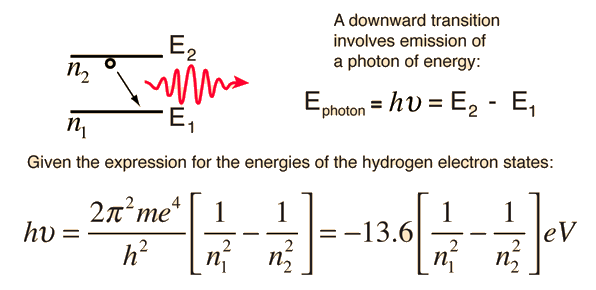
This is often expressed in terms of the inverse wavelength or "wave number" as follows:
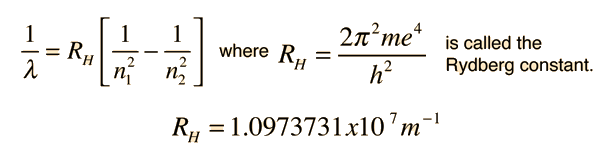
Going on, we can calculate the net energy (kinetic plus potential) of an electron in the 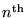 Bohr orbital of the H atom:
Bohr orbital of the H atom:
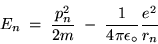
Answered by | 15 May, 2012, 11:49: AM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by ee7511641 | 13 Jan, 2024, 03:37: PM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by o230397 | 23 Sep, 2023, 02:48: PM
CBSE 11-science - Chemistry
Asked by shrreya27harshitha | 17 Jul, 2022, 04:15: PM
CBSE 11-science - Chemistry
Asked by habibakhatoon112 | 15 Jul, 2022, 09:14: PM
CBSE 11-science - Chemistry
Asked by deba.biswas561 | 14 Jun, 2022, 08:07: AM
CBSE 11-science - Chemistry
Asked by advssdrall | 12 Jan, 2022, 05:12: AM
CBSE 11-science - Chemistry
Asked by ks1221516 | 14 Nov, 2021, 06:46: PM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 22 Oct, 2021, 07:29: PM









