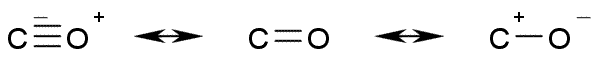CBSE Class 10 Answered
how is the carbon monoxide bond is formed?[explain with figure ] also show the sahring of electrons
Asked by | 14 Apr, 2008, 09:01: PM
Resonating structures of Carbon Monoxide
The molecule's bond length is consistent with a partial triple bond. The molecule has a small dipole moment and can be represented by three resonance structures.The leftmost resonance form is the most important as inferred by reactions of carbon monoxide.The structures above explain the sharing of electrons between carbon and oxygen.Further explaination requires use of molecular orbital theory which is beyond the scope of 10th standard.
Answered by | 13 Jun, 2008, 03:39: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by kamalapallysudha17 | 25 Mar, 2024, 07:52: PM
CBSE 10 - Chemistry
Asked by sagarmishra | 04 Mar, 2024, 09:50: AM
CBSE 10 - Chemistry
Asked by 09.10bjanvhijadhav | 02 Mar, 2024, 08:22: AM
CBSE 10 - Chemistry
Asked by prassanna.j | 01 Mar, 2024, 11:59: AM
CBSE 10 - Chemistry
Asked by ritik9897022 | 05 Feb, 2024, 09:42: PM
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by amanazeez6 | 14 Dec, 2023, 01:10: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by susrisangita792 | 17 Nov, 2023, 08:27: PM