CBSE Class 11-science Answered
How is sodium different from potassium?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
a) Sodium is heavier than potassium due to increase in atomic size of potassium, which makes its density lower.
b) Sodium forms peroxides, which are colorless and diamagnetic with oxygen while potassium forms superoxide.
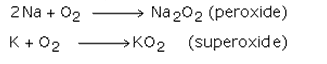
c) The ion K+ is larger and has a very weak positive field. Thus this ions stabilizes a bigger superoxide O2- anion and form superoxides which are colored and paramagnetic.
d) The salts of alkali metals are the most ionic salts known. Although lithium is an alkali yet its compounds, particularly halides, are slightly covalent in nature.
b) Sodium forms peroxides, which are colorless and diamagnetic with oxygen while potassium forms superoxide.
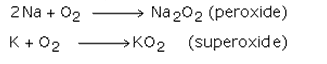
c) The ion K+ is larger and has a very weak positive field. Thus this ions stabilizes a bigger superoxide O2- anion and form superoxides which are colored and paramagnetic.
d) The salts of alkali metals are the most ionic salts known. Although lithium is an alkali yet its compounds, particularly halides, are slightly covalent in nature.
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:49: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:53: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 03:32: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 03:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 03:13: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:54: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM





