CBSE Class 10 Answered
how is SO4 anion formed?
Asked by | 17 May, 2008, 12:43: PM
![]()
Fig1. Fig.2
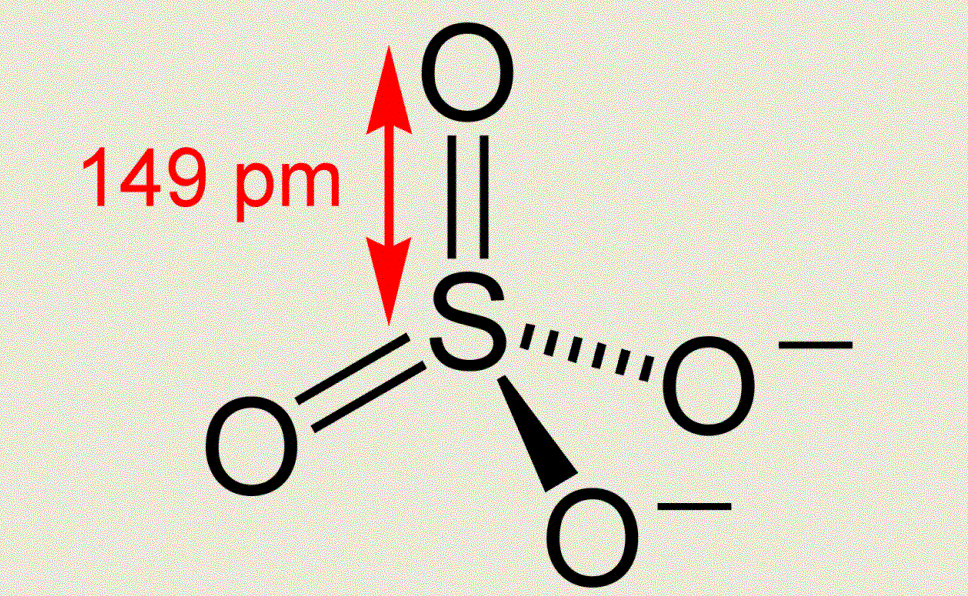
The sulfur atom in sulfate ion is sp3 hybridized. This is dictated by the fact that it needs to form bonds to four oxygen atoms in a molecule having symmetry. In addition, electrons from the non-bonding p atomic orbitals on oxygen are able to form p bonds with the sulfur atom's empty d orbitals as shown in Figure 2. These p bonds are "interchanged" by resonance, and the net result is similar to that shown in the resonating Lewis structures of Figure 3.
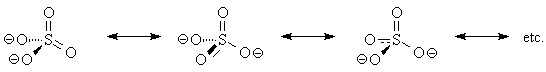 Fig.3
Fig.3
Answered by | 13 Jun, 2008, 08:12: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by kamalapallysudha17 | 25 Mar, 2024, 07:52: PM
CBSE 10 - Chemistry
Asked by sagarmishra | 04 Mar, 2024, 09:50: AM
CBSE 10 - Chemistry
Asked by 09.10bjanvhijadhav | 02 Mar, 2024, 08:22: AM
CBSE 10 - Chemistry
Asked by prassanna.j | 01 Mar, 2024, 11:59: AM
CBSE 10 - Chemistry
Asked by ritik9897022 | 05 Feb, 2024, 09:42: PM
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by amanazeez6 | 14 Dec, 2023, 01:10: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by susrisangita792 | 17 Nov, 2023, 08:27: PM