ICSE Class 10 Answered
How does use of silver nitrate as an electrolyte in electroplating of silver on any article, increase the speed of deposition?Explain.
Asked by | 19 Oct, 2013, 01:03: PM
Rate of dissociation is high in the case of silver nitrate as it is an ionic molecule where as Sodium argentocyanide or potassium argentocyanide are Coordination complex molecules. During the dissociation of Sodium argentocyanide (or) potassium argentocyanide, the rate gets slow down as silver is present inside the complex whereas in case of silver nitrate, silver ions are released at a faster rate.
AgNO?
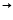 Ag? + NO??
Ag? + NO??Na[Ag(CN)?]
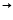 Na? + Ag? + 2CN?
Na? + Ag? + 2CN?
Answered by | 24 Oct, 2013, 10:38: AM
Concept Videos
ICSE 10 - Chemistry
Asked by naaimahhaq | 17 Feb, 2024, 10:54: AM
ICSE 10 - Chemistry
Asked by sanskritimshra2008 | 01 Feb, 2024, 07:38: PM
ICSE 10 - Chemistry
Asked by benitojohnson2007 | 10 Aug, 2023, 08:00: PM
ICSE 10 - Chemistry
Asked by Himadri | 28 Jul, 2020, 02:27: PM
ICSE 10 - Chemistry
Asked by saibaba1069 | 24 Jul, 2020, 08:46: PM
ICSE 10 - Chemistry
Asked by amarjeetsingh0811.10sdatl | 09 Jun, 2020, 09:16: AM
ICSE 10 - Chemistry
Asked by gupta.eshan73 | 20 Feb, 2020, 12:37: PM
ICSE 10 - Chemistry
Asked by arpitt682 | 24 Dec, 2019, 10:45: AM
ICSE 10 - Chemistry
Asked by ash1080ekhar | 22 Dec, 2019, 09:19: PM
ICSE 10 - Chemistry
Asked by arpitt682 | 23 Aug, 2019, 03:18: PM






