CBSE Class 11-science Answered
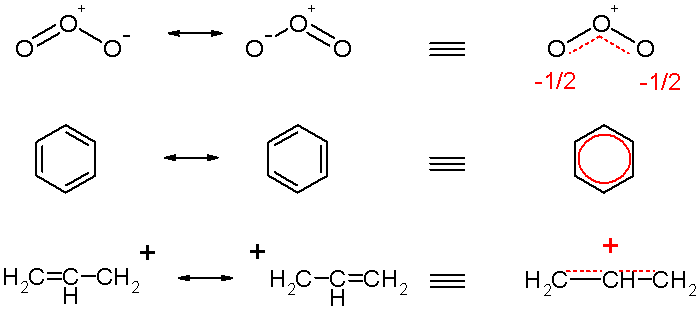
Resonance hybrids are always more stable than any of the canonical structures would be, if they existed[1]. The delocalization of the electrons lowers the orbital energies, imparting this stability. The resonance in benzene gives rise to the property of aromaticity. The gain in stability of the resonance hybrid over the most stable of the (non-existent) canonical structures is called the resonance energy. A canonical structure that is lower in energy makes a relatively greater contribution to the resonance hybrid, or the actual picture of the molecule. In fact, resonance energy, and consequently stability, increase with the number of canonical structures possible, especially when these (non-existent) structures are equal in energy.