CBSE Class 11-science Answered
How does the density of a substance depend on temperature? Explain with a mathematical formula?
Asked by shobhit_dc | 23 Jul, 2011, 11:33: PM
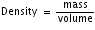
Density does not change as long as temperature remains the same. For every substance, the number of particles in a given volume remain constant if the temperature is kept constant. As the temperature is increased, the particles move more quickly and farther apart, thus increasing the substance's volume. When this happens, the density (which is the mass to volume ratio) decreases because mass remains constant, but volume increases.
Answered by | 24 Jul, 2011, 01:32: AM
Concept Videos
CBSE 11-science - Physics
Asked by manjushri30nov | 31 Dec, 2023, 07:48: PM
CBSE 11-science - Physics
Asked by abhishekmadheshiya708 | 22 Dec, 2023, 01:38: PM
CBSE 11-science - Physics
Asked by ankitvariya1000 | 29 Mar, 2022, 11:19: AM
CBSE 11-science - Physics
Asked by jatinsahu37 | 27 Mar, 2022, 04:45: PM
CBSE 11-science - Physics
Asked by parineetsandhu3232 | 11 Mar, 2022, 05:49: PM
CBSE 11-science - Physics
Asked by arjunsah797 | 01 Mar, 2022, 03:01: PM
CBSE 11-science - Physics
Asked by sanjanapujar80 | 12 Jun, 2021, 10:29: AM
CBSE 11-science - Physics
Asked by akpdbbhhs | 14 May, 2021, 12:51: PM
CBSE 11-science - Physics
Asked by vikranthpoluparthi1 | 04 May, 2021, 12:30: PM
CBSE 11-science - Physics
Asked by shubhamgoyal1722 | 15 Dec, 2020, 11:18: AM







