CBSE Class 10 Answered
How does carboxylic acid gets oxidized?
Asked by Sachin verma | 24 Feb, 2011, 07:51: PM
Dear Student
Because it is already in a high oxidation state, further oxidation removes the carboxyl carbon as carbon dioxide. Depending on the reaction conditions, the oxidation state of the remaining organic structure may be higher, lower or unchanged. The following reactions are all examples of decarboxylation (loss of CO2). In the first, bromine replaces the carboxyl group, so both the carboxyl carbon atom and the remaining organic moiety are oxidized.
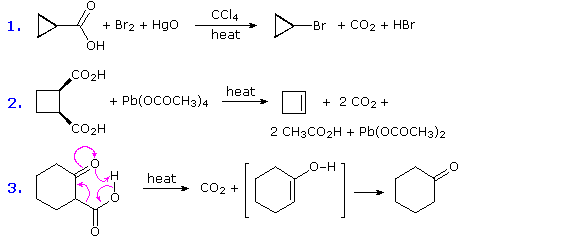
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 24 Feb, 2011, 04:05: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by agarwalkrishnam98 | 01 Oct, 2023, 08:28: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:01: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM











