CBSE Class 11-science Answered
How do we get acetate free radical during Kolbe's electrlytic preparation of ethane from sodium acetate?
Asked by | 25 Sep, 2012, 10:17: PM
This reaction is a electrochemical decarboxylation of the salts of carboxylic acid which produces radicals. This reaction is also called as decarboxylative dimerisation, since it proceeds with radical reaction mechanism. This reaction is used in synthesis of symmetrical dimers. It can also be employed for a mixture of carboxylic acid in order to furnish unsymmetrical dimers.
For example, consider the following reaction

Free radicals always undergo disproportionation or auto oxidation and dimerization. Because of these reaction in kolbe electrolysis, the mixture of hydrocarbons are formed
Answered by | 27 Sep, 2012, 10:04: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
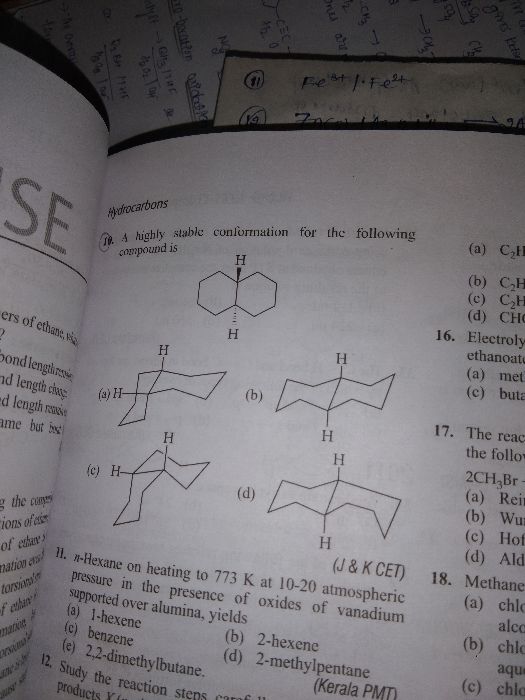
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM