CBSE Class 11-science Answered
how do we determine the electron density regions?
And why is the no.of electron density regions of triodide anion 5 pls explain
Asked by SAI GANESH BEHARA | 21 Feb, 2011, 06:23: AM
Dear Student
The electron density is simply deduced from the atomic density as follows :
if ne denotes the electron density and na the atomic density then :
ne= z na
with z being the number of valence electron per atom.
If you want to know the regions of electron density, follow the simple rules:
a) Draw the Lewis structure for the molecule or ion.
b) Count the total number of regions of high electron density (bonding and unshared electron pairs) around the central atom.
(i) Double and triple bonds count as ONE REGION OF HIGH ELECTRON DENSITY.
- An unpaired electron counts as ONE REGION OF HIGH ELECTRON DENSITY.
- For molecules or ions that have resonance structures, you may use any one of the resonance structures.
For example: ![]() has 2 regions of high electron density.
has 2 regions of high electron density.
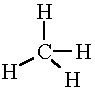 has 4 regions of high electron density.
has 4 regions of high electron density.
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 21 Feb, 2011, 10:45: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM






