CBSE Class 10 Answered
HELP
Asked by | 20 Jan, 2009, 02:51: PM
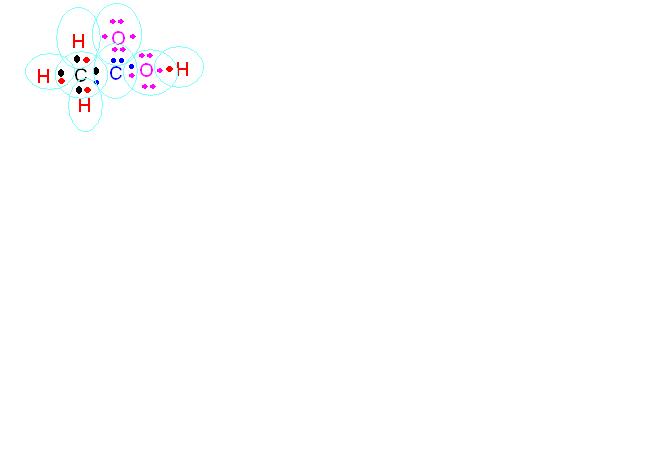
The molecular formula of Ethanoic acid is CH3COOH.
The atomic number of C is 6 . So,its electronic configuration is 2,4. The valence electrons are 4.
The atomic number of H is 1 . So,its electronic configuration is 1. The valence electrons are 1.
The atomic number of O is 8 . So,its electronic configuration is 2,6. The valence electrons are 6.
So, its electron dot structure is as follows:
Answered by | 21 Jan, 2009, 04:16: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by agarwalkrishnam98 | 01 Oct, 2023, 08:28: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:01: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM