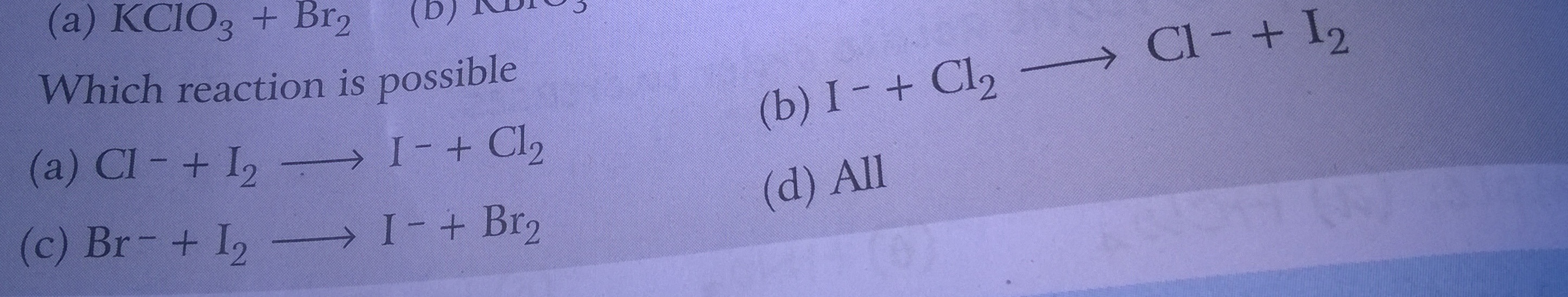CBSE Class 11-science Answered
Hello there, Statement in my book reads as " a reaction in which two atoms or molecules combine together to form a third molecule is a combination reaction, and for this reaction to be redox reaction, either one of A and B must be in elemental form.
A+B=C
Why underlined text is an essential requirement if we want to have a redox combination reaction,
Please Exlain with examples why can't we have both A and B in non-elemental form.
Asked by Nirbhay | 01 Nov, 2014, 11:23: PM
Dear oneworld191@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
For a combination reaction to be a redox reaction, either A or B or both A and B must be in elemental form. If they both are in non-elemental form as shown in below example:
2Cu2O(s) + Cu2S(s) → 6Cu(s) + SO2(g) (It is a redox reaction)
then the reaction will no longer remain as combination reaction. Due to this reason either A or B or both A and B must be in elemental form. It is a mandatory condition.Regards
Topperlearning Team.
Answered by Arvind Diwale | 03 Nov, 2014, 01:22: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by jaip83491 | 24 Jan, 2021, 10:43: AM
CBSE 11-science - Chemistry
Asked by sulaikhasulu393 | 07 Jun, 2020, 10:51: PM
CBSE 11-science - Chemistry
Asked by debjit_dm | 04 May, 2020, 03:08: PM
CBSE 11-science - Chemistry
Asked by Balbir | 30 Jun, 2019, 03:45: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 05 Sep, 2018, 01:32: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 09 Aug, 2018, 04:52: PM
CBSE 11-science - Chemistry
Asked by dineshchem108 | 17 Jul, 2018, 06:02: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 09:55: PM
CBSE 11-science - Chemistry
Asked by Balbir | 19 Jun, 2018, 06:36: PM